Here are the essential concepts you must grasp in order to answer the question correctly.
Hydrogen Bonding
Hydrogen bonding is a type of intermolecular force that occurs when a hydrogen atom covalently bonded to a highly electronegative atom, such as oxygen or nitrogen, interacts with another electronegative atom. In the case of ethyl alcohol and water, the hydrogen atoms of the hydroxyl group (-OH) in ethyl alcohol can form hydrogen bonds with the oxygen atoms of water molecules, significantly influencing the solubility and properties of the solution.
Recommended video:
Molecular Structure of Ethyl Alcohol
Ethyl alcohol (CH3CH2OH) consists of a two-carbon chain with a hydroxyl (-OH) functional group. The presence of the hydroxyl group makes ethyl alcohol polar, allowing it to interact favorably with polar solvents like water. Understanding the molecular structure is crucial for predicting how many hydrogen bonds can form between ethyl alcohol and water.
Recommended video:
Rules for Naming Alcohols
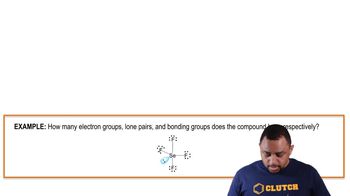
Lone Pairs of Electrons
Lone pairs of electrons are pairs of valence electrons that are not involved in bonding and are localized on a single atom. In the context of hydrogen bonding, the lone pairs on the oxygen atom of water are essential for forming hydrogen bonds with the hydrogen atom of the hydroxyl group in ethyl alcohol. Recognizing the role of lone pairs helps in visualizing and sketching the hydrogen bonding interactions.
Recommended video:
Electron Groups, Lone Pairs, and Bonding Groups Example
 Verified step by step guidance
Verified step by step guidance