For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (i)
Ch.9 - Molecular Geometry and Bonding Theories
Chapter 9, Problem 11b3
For each of these contour representations of molecular orbitals, identify (b) the type of MO (s or p) (iii)

 Verified step by step guidance
Verified step by step guidance1
Observe the contour representation of the molecular orbital in the image.
Identify the shape of the molecular orbital. In this case, it appears to be elongated along one axis, indicating a p-orbital.
Note the presence of a nodal plane (a region where the probability of finding an electron is zero) between the positive and negative regions, which is characteristic of p-orbitals.
Determine the type of molecular orbital (MO) based on the shape and nodal plane. Since it has a nodal plane and is elongated, it is a p-type MO.
Conclude that the molecular orbital shown in the image is a p-type molecular orbital.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Orbitals (MOs)
Molecular orbitals are formed by the combination of atomic orbitals when atoms bond together. They can be classified as bonding, antibonding, or non-bonding, depending on the phase relationship of the combining atomic orbitals. Understanding the shape and energy of these orbitals is crucial for predicting the behavior of molecules in chemical reactions.
Recommended video:
Guided course

Molecular Orbital Theory
Types of Atomic Orbitals
Atomic orbitals are regions in an atom where there is a high probability of finding electrons. The main types are s and p orbitals. S orbitals are spherical and can hold up to two electrons, while p orbitals are dumbbell-shaped and can hold up to six electrons across three orientations (px, py, pz). Identifying the type of molecular orbital often involves recognizing these shapes.
Recommended video:
Guided course

Atomic Orbitals Example
Contour Representation
Contour representations visually depict the regions of positive and negative phase in molecular orbitals. The areas where the orbital has a positive phase are often shown in one color, while negative phases are shown in another. This representation helps in understanding the electron density distribution and the bonding characteristics of the molecule.
Recommended video:
Guided course
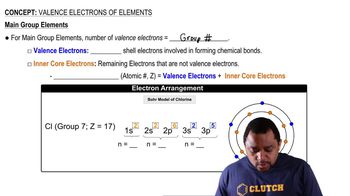
Main Group Elements Valence Electrons
Related Practice
Textbook Question
551
views
Textbook Question
For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)
345
views
Textbook Question
For each of these contour representations of molecular orbitals, identify (a) the atomic orbitals (s or p) used to construct the MO (iii)
375
views
Textbook Question
The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. ii.
364
views
Textbook Question
For each of these contour representations of molecular orbitals, identify (b) the type of MO (s or p) (i)
272
views
Textbook Question
For each molecule (a)–(f), indicate how many different electron-domain geometries are consistent with the molecular geometry shown. a.
765
views
