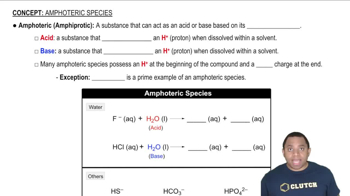
Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic substance azobenzene, C12H10N2. A closely related substance is hydrazobenzene, C12H12N2. The Lewis structures of these two substances are
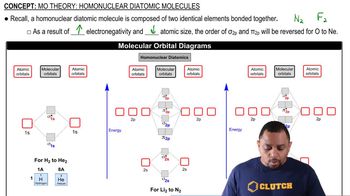
(Recall the shorthand notation used for benzene.) (b) How many unhybridized atomic orbitals are there on the N and the C atoms in each of the substances? How many unhybridized atomic orbitals are there on the N and the C atoms in hydrazobenzene?