The lobes of which d orbitals point directly between the ligands in b. tetrahedral geometry?
For a given metal ion and set of ligands, is the crystal-field splitting energy larger for a tetrahedral or an octahedral geometry?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
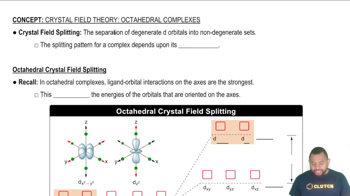
Key Concepts
Crystal Field Theory

Tetrahedral vs. Octahedral Geometry

Crystal Field Splitting Energy (Δ)
As shown in Figure 23.26, the d-d transition of [Ti(H2O)6]³⁺ produces an absorption maximum at a wavelength of about 500 nm .
a. What is the magnitude of ∆ for [Ti(H2O)6]³⁺ in kJ/mol?
b. How would the magnitude of ∆ change if the H2O ligands in [Ti(H2O)6]]³⁺ were placed with NH3 ligands?
A classmate says, “A weak-field ligand usually means the complex is high spin.” Is your classmate correct? Explain.
Complete the exercises below. Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
a. [VCl6]3–,
b. [FeF6]3– (a high-spin complex),
Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
d. [NiCl4]2+ (tetrahedral),
Sketch the structure of the complex in each of the following compounds and give the full compound name:
a. cis-[Co(NH3)4(H2O)2] (NO3)2
