A classmate says, “A weak-field ligand usually means the complex is high spin.” Is your classmate correct? Explain.
Ch.23 - Transition Metals and Coordination Chemistry
Chapter 23, Problem 64d
Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
d. [NiCl4]2+ (tetrahedral),
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the metal ion and its oxidation state. In this case, it's Ni2+. The electron configuration of Ni is [Ar] 3d8 4s2. Since it's in the +2 oxidation state, it loses the 4s electrons, leaving it with [Ar] 3d8.
Step 2: Identify the geometry of the complex. In this case, it's tetrahedral. In a tetrahedral complex, the d orbitals split into two energy levels: the higher energy level (e) consists of the dxy, dxz, and dyz orbitals, and the lower energy level (t2) consists of the dz2 and dx2-y2 orbitals.
Step 3: Fill in the electrons according to Hund's rule, which states that electrons fill each orbital singly before any orbital gets a second electron. Since Ni2+ has 8 d electrons, fill in the 5 lower energy orbitals first, then the 3 higher energy orbitals.
Step 4: Draw the crystal-field energy-level diagram. The lower energy level (t2) should be filled with 5 electrons and the higher energy level (e) should be filled with 3 electrons.
Step 5: Remember that the energy difference between the two levels in a tetrahedral complex is smaller than in an octahedral complex, due to the different orientations of the ligands in relation to the d orbitals.

Verified Solution
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Crystal Field Theory
Crystal Field Theory (CFT) explains the electronic structure of transition metal complexes by considering the interaction between the metal ion's d-orbitals and the electric fields produced by surrounding ligands. In this theory, the degeneracy of d-orbitals is lifted, leading to different energy levels based on the geometry of the complex, such as octahedral or tetrahedral arrangements.
Recommended video:
Guided course

The study of ligand-metal interactions helped to form Ligand Field Theory which combines CFT with MO Theory.
Tetrahedral Complexes
In tetrahedral complexes, the arrangement of ligands around the central metal ion creates a specific geometry where the d-orbitals split into two sets: the lower-energy e set (dxy, dyz, dzx) and the higher-energy t2 set (dx2-y2, dz2). This splitting is less pronounced than in octahedral complexes, resulting in a smaller energy difference between the orbitals, which influences electron placement and the overall stability of the complex.
Recommended video:
Guided course
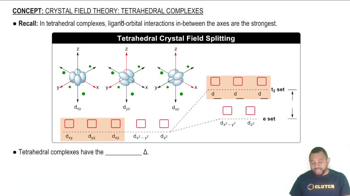
The crystal field splitting pattern for tetrahedral complexes has the d orbitals in between the axes as having the higher energy.
Electron Configuration and Placement
The electron configuration of a transition metal ion determines how electrons are distributed among the available d-orbitals. For [NiCl4]2+, nickel is in the +2 oxidation state, leading to a d8 configuration. In a tetrahedral field, the electrons will fill the lower-energy e orbitals first, followed by the higher-energy t2 orbitals, which is crucial for accurately drawing the crystal-field energy-level diagram.
Recommended video:
Guided course

Electron Configuration Example
Related Practice
Textbook Question
101
views
Textbook Question
For a given metal ion and set of ligands, is the crystal-field splitting energy larger for a tetrahedral or an octahedral geometry?
130
views
Textbook Question
Complete the exercises below. Draw the crystal-field energy-level diagrams and show the placement of electrons for each of the following complexes:
a. [VCl6]3–,
b. [FeF6]3– (a high-spin complex),
Textbook Question
Sketch the structure of the complex in each of the following compounds and give the full compound name:
a. cis-[Co(NH3)4(H2O)2] (NO3)2
109
views
Textbook Question
Sketch the structure of the complex in each of the following compounds and give the full compound name:
b. Na2[Ru(H2O)Cl5]
108
views
Textbook Question
Sketch the structure of the complex in each of the following compounds and give the full compound name:
c. trans-NH3[Co(C2O4)2(H2O)2]
107
views
