An AB5 molecule adopts the geometry shown here. (c) Suppose the B atoms are halogen atoms. Of which group in the periodic table is atom A a member: (i) Group 15, (ii) Group 16, (iii) Group 17, (iv) Group 18, or (v) More information is needed?
Which of the following statements about hybrid orbitals is or are true? (i) After an atom undergoes sp hybridization, there is one unhybridized p orbital on the atom, (ii) Under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle, and (iii) The angle between the large lobes of sp3 hybrids is 109.5°.

Verified Solution
Key Concepts
Hybridization

Geometry of Hybrid Orbitals
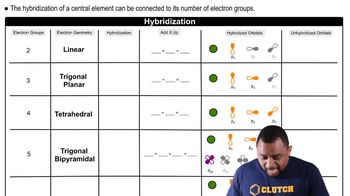
Unhybridized Orbitals

The O¬H bond lengths in the water molecule 1H2O2 are 96 pm, and the H¬O¬H angle is 104.5°. The dipole moment of the water molecule is 1.85 D. (b) Calculate the magnitude of the bond dipole of the O¬H bonds. (Note: You will need to use vector addition to do this.)
a) Predict the electron-domain geometry around the central S atom in SF2, SF4, and SF6.
Sodium azide is a shock-sensitive compound that releases N2 upon physical impact. The compound is used in automobile airbags. The azide ion is N3-. (a) Draw the Lewis structure of the azide ion that minimizes formal charge (it does not form a triangle). Is it linear or bent?
Sodium azide is a shock-sensitive compound that releases N2 upon physical impact. The compound is used in automobile airbags. The azide ion is N3-. (b) State the hybridization of the central N atom in the azide ion.
In ozone, O3, the two oxygen atoms on the ends of the molecule are equivalent to one another. (d) How many electrons are delocalized in the p system of ozone?
