Textbook Question
For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)
345
views
 Verified step by step guidance
Verified step by step guidance

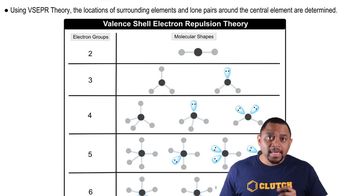

For each of these contour representations of molecular orbitals, identify (c) whether the MO is bonding or antibonding (i)
(a) An AB2 molecule is linear. How many nonbonding electron pairs are around the A atom from this information?
(b) How many nonbonding electrons surround the Xe in XeF2?
(a) Boron trichloride 1BCl32 and the carbonate ion 1CO3 2- 2 are both described as trigonal. What does this indicate about their bond angles?
(b) The PCl3 molecule is trigonal pyramidal, while ICl3 is T-shaped. Which of these molecules is flat?