Here are the essential concepts you must grasp in order to answer the question correctly.
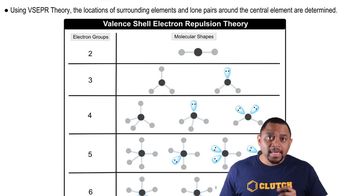
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influence the shape. For example, PCl3 has a trigonal pyramidal shape due to one lone pair, while ICl3 has a T-shaped geometry due to three lone pairs.
Recommended video:
Molecular Geometry with Two Electron Groups
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of molecules based on the repulsion between electron pairs. According to VSEPR, electron pairs will arrange themselves to minimize repulsion, leading to specific molecular shapes. This theory helps explain why PCl3 is trigonal pyramidal and ICl3 is T-shaped.
Recommended video:
Molecular Shapes and VSEPR
Planarity in Molecules
Planarity in molecules refers to the arrangement of atoms in a single plane. A molecule is considered flat if all its atoms lie in the same plane, which is often the case for molecules with specific geometries like trigonal planar or linear. In this context, ICl3 is T-shaped and has a flat arrangement, while PCl3 is not flat due to its trigonal pyramidal shape.
Recommended video:
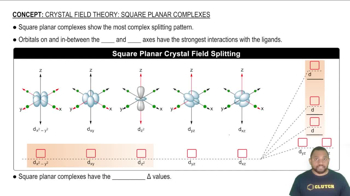
Square planar complexes show the most complex splitting pattern.
 Verified step by step guidance
Verified step by step guidance