Textbook Question
(a) Boron trichloride 1BCl32 and the carbonate ion 1CO3 2- 2 are both described as trigonal. What does this indicate about their bond angles?
405
views
 Verified step by step guidance
Verified step by step guidance

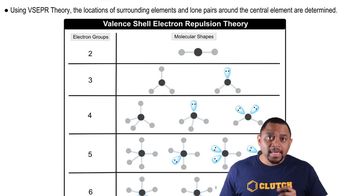
(a) Boron trichloride 1BCl32 and the carbonate ion 1CO3 2- 2 are both described as trigonal. What does this indicate about their bond angles?
(b) The PCl3 molecule is trigonal pyramidal, while ICl3 is T-shaped. Which of these molecules is flat?
(b) An AB4 molecule has two lone pairs of electrons on the A atom (in addition to the four B atoms). What is the electron-domain geometry around the A atom?
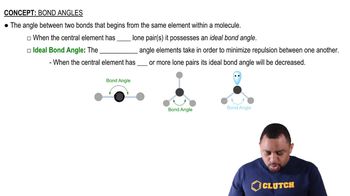
Would you expect the nonbonding electron-pair domain in NCl3 to be greater or smaller in size than the corresponding one in PCl3?