The substance chlorine monoxide, ClO(g), is important in atmospheric processes that lead to depletion of the ozone layer. The ClO molecule has an experimental dipole moment of 1.24 D, and the Cl—O bond length is 160 pm. (c) Using formal charges as a guide, propose the dominant Lewis structure for the molecule. (g), is important in atmospheric processes that lead to depletion of the ozone layer. The ClO molecule has an experimental dipole moment of 1.24 D, and the Cl—O bond length is 160 pm. (d) The anion ClO exists. What is the formal charge on the Cl for the best Lewis structure for ClO-?

(c) The measured dipole moment of BrCl is 0.57 D. If you assume the bond length in BrCl is the sum of the atomic radii, what are the partial charges on the atoms in BrCl using the experimental dipole moment?
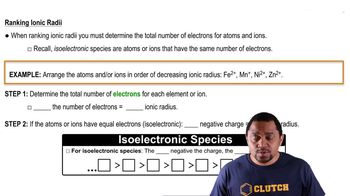
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Dipole Moment

Partial Charges

Bond Length and Atomic Radii
(a) Using the electronegativities of Br and Cl, estimate the partial charges on the atoms in the Br¬Cl molecule.
(b) Using these partial charges and the atomic radii given in Figure 7.8, estimate the dipole moment of the molecule.
A major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (b) Which element in NaAlH4 is the most electronegative? Which one is the least electronegative? Which element in NaAlH4 is the least electronegative?
A major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (c) Based on electronegativity differences, predict the identity of the polyatomic anion. Draw a Lewis structure for this ion.
A major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (d) What is the formal charge on hydrogen in the polyatomic ion?
