A major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (b) Which element in NaAlH4 is the most electronegative? Which one is the least electronegative? Which element in NaAlH4 is the least electronegative?
Although I3- is a known ion, F3- is not. (b) One of your classmates says that F3- does not exist because F is too electronegative to make bonds with another atom. Give an example that proves your classmate is wrong.
Recommended similar problem, with video answer:

Verified Solution
Key Concepts
Electronegativity

Covalent Bonding
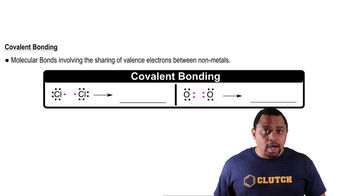
Polyatomic Ions

A major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (c) Based on electronegativity differences, predict the identity of the polyatomic anion. Draw a Lewis structure for this ion.
A major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (d) What is the formal charge on hydrogen in the polyatomic ion?
Although I3- is a known ion, F3- is not. (c) Another classmate says F3- does not exist because it would violate the octet rule. Is this classmate possibly correct?
Calculate the formal charge on the indicated atom in each of the following molecules or ions: (a) the central oxygen atom in O3 (b) phosphorus in PF6- (c) nitrogen in NO2 (d) iodine in ICl3 (e) chlorine in HClO4 (hydrogen is bonded to O).
The hypochlorite ion, ClO-, is the active ingredient in bleach. The perchlorate ion, ClO4-, is a main component of rocket propellants. Draw Lewis structures for both ions. (b) What is the formal charge of Cl in the perchlorate ion, assuming the Cl—O bonds are all single bonds?
