(d) Lithium is not nearly as abundant as sodium. If sodium ion batteries were developed that function in the same manner as lithium ion batteries, do you think 'sodium cobalt oxide' would still work as the electrode material? Explain.
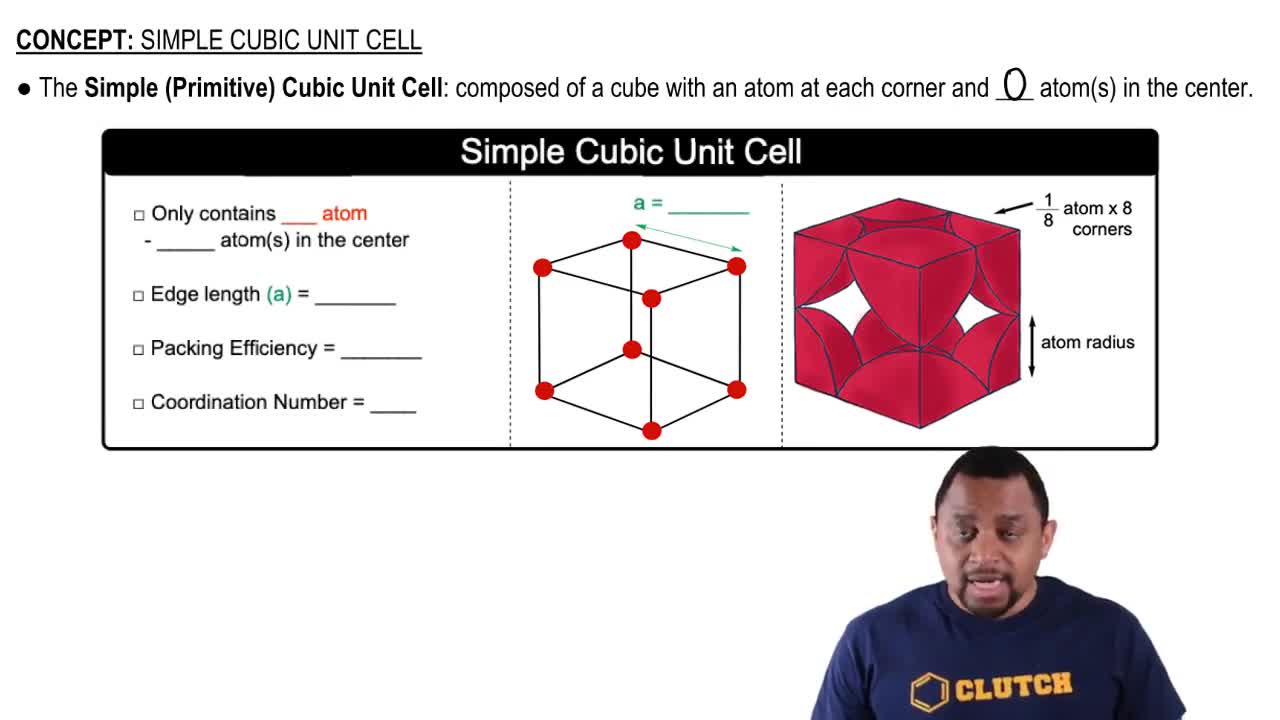
The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl:
(c) The density of SrO is 5.10 g>cm3. Given your answer to part (b), how many formula units of SrO are contained in the cube shown here?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Ionic Compounds

Density and Volume Calculations

Unit Cell and Formula Units
The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl: (a) Write a balanced equation for the formation of SrO(s) from its elements.
The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl:
(b) Based on the ionic radii in Figure 7.8, predict the length of the side of the cube in the figure (the distance from the center of an atom at one corner to the center of an atom at a neighboring corner).
Explain the variation in the ionization energies of carbon, as displayed in this graph:
In the chemical process called electron transfer, an electron is transferred from one atom or molecule to another. (We will talk about electron transfer extensively in Chapter 20.) A simple electron transfer reaction is A(g) + A(g) → A+(g) + A-(g) For a representative nonmetal such as chlorine, is this process exothermic?
(a) Use orbital diagrams to illustrate what happens when an oxygen atom gains two electrons
