The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl: (a) Write a balanced equation for the formation of SrO(s) from its elements.
Explain the variation in the ionization energies of carbon, as displayed in this graph:
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
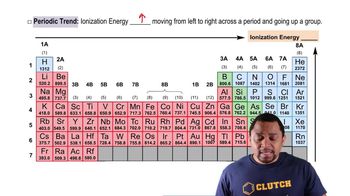
Ionization Energy

Trends in Ionization Energy
Electron Configuration

The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl:
(b) Based on the ionic radii in Figure 7.8, predict the length of the side of the cube in the figure (the distance from the center of an atom at one corner to the center of an atom at a neighboring corner).
The ionic substance strontium oxide, SrO, forms from the reaction of strontium metal with molecular oxygen. The arrangement of the ions in solid SrO is analogous to that in solid NaCl:
(c) The density of SrO is 5.10 g>cm3. Given your answer to part (b), how many formula units of SrO are contained in the cube shown here?
In the chemical process called electron transfer, an electron is transferred from one atom or molecule to another. (We will talk about electron transfer extensively in Chapter 20.) A simple electron transfer reaction is A(g) + A(g) → A+(g) + A-(g) For a representative nonmetal such as chlorine, is this process exothermic?
(a) Use orbital diagrams to illustrate what happens when an oxygen atom gains two electrons
