The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (a) Based on these data, what is the predicted As ¬ Cl bond length in arsenic trichlo- ride, AsCl3, in which each of the three Cl atoms is bonded to the As atom?
Ch.7 - Periodic Properties of the Elements
Chapter 7, Problem 87a
Elements in group 7A in the periodic table are called the halogens; elements in group 6A are called the chalcogens. (a) What is the most common oxidation state of the chalcogens compared to the halogens?
 Verified step by step guidance
Verified step by step guidance1
Identify the elements in group 6A (chalcogens) and group 7A (halogens) of the periodic table.
Recall that the most common oxidation state for an element is often related to the number of electrons needed to achieve a full outer shell.
For chalcogens (group 6A), note that they have six valence electrons and need two more electrons to achieve a full octet, leading to a common oxidation state of -2.
For halogens (group 7A), note that they have seven valence electrons and need one more electron to achieve a full octet, leading to a common oxidation state of -1.
Compare the most common oxidation states: chalcogens typically have an oxidation state of -2, while halogens typically have an oxidation state of -1.

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation States
Oxidation states indicate the degree of oxidation of an atom in a compound, reflecting the number of electrons lost, gained, or shared. For chalcogens (group 6A), the most common oxidation state is -2, as they typically gain two electrons to achieve a stable electron configuration. In contrast, halogens (group 7A) usually exhibit a -1 oxidation state, as they tend to gain one electron.
Recommended video:
Guided course

Oxidation Numbers
Periodic Table Groups
The periodic table is organized into groups (columns) that share similar chemical properties. Group 6A, the chalcogens, includes elements like oxygen and sulfur, which are known for forming compounds with a -2 oxidation state. Group 7A, the halogens, includes elements like fluorine and chlorine, which are highly reactive and typically have a -1 oxidation state due to their tendency to gain one electron.
Recommended video:
Guided course
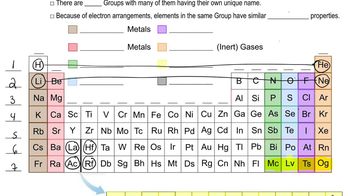
Periodic Table: Group Names
Chemical Reactivity
Chemical reactivity refers to how readily an element undergoes chemical reactions, influenced by its electron configuration. Halogens are more reactive than chalcogens because they are one electron short of a full valence shell, making them eager to gain that electron. Chalcogens, while also reactive, are less so than halogens, as they are two electrons away from achieving a stable configuration.
Recommended video:
Guided course
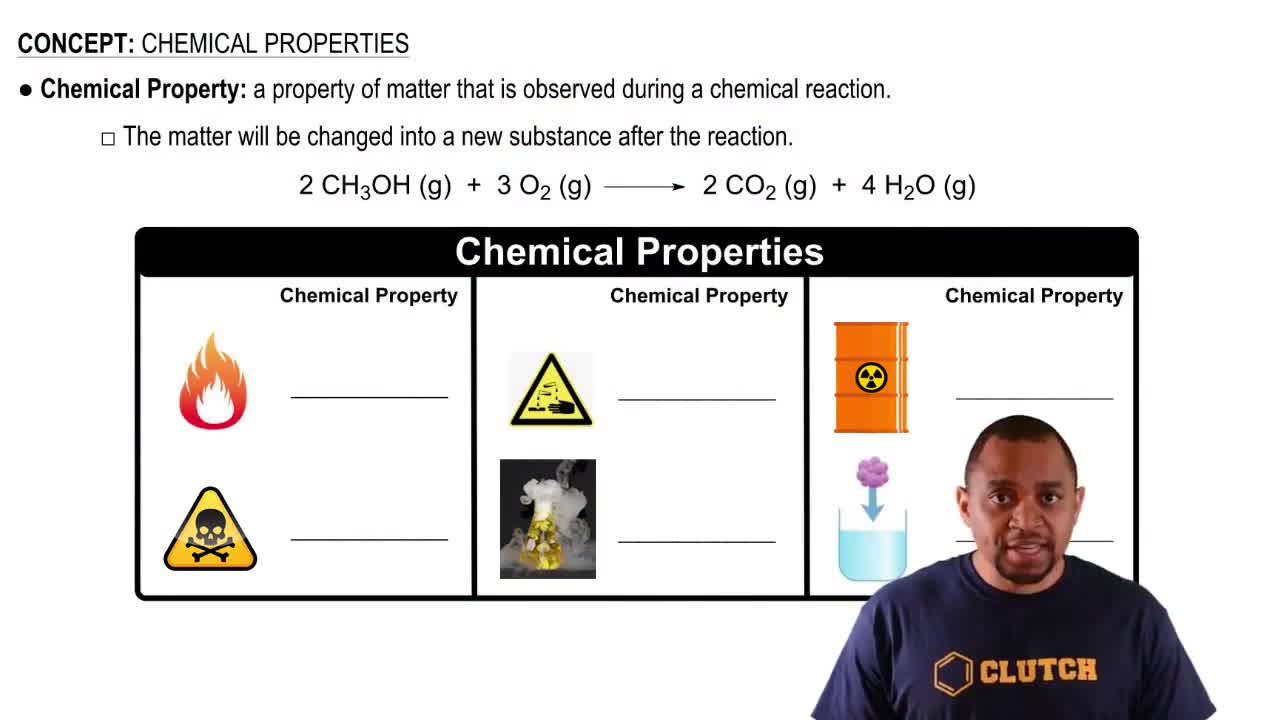
Chemical Properties
Related Practice
Textbook Question
1111
views
Textbook Question
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?
778
views
Textbook Question
The following observations are made about two hypothetical
elements A and B: The A¬A and B¬B bond lengths in the
elemental forms of A and B are 236 and 194 pm, respectively.
A and B react to form the binary compound AB2, which has
a linear structure (that is B-A-B = 180°). Based on these
statements, predict the separation between the two B nuclei
in a molecule of AB2.
701
views
Textbook Question
Note from the following table that there is a significant increase
in atomic radius upon moving from Y to La, whereas
the radii of Zr to Hf are the same. Suggest an explanation for
this effect.
Atomic Radii (pm)
Sc 170 Ti 160
Y 190 Zr 175
La 207 Hf 175
970
views
Textbook Question
(c) Will the lithium cobalt oxide cathode expand or contract as lithium ions are inserted?
529
views
Textbook Question
(d) Lithium is not nearly as abundant as sodium. If sodium ion batteries were developed that function in the same manner as lithium ion batteries, do you think 'sodium cobalt oxide' would still work as the electrode material? Explain.
504
views
