Textbook Question
Which of these spheres represents F, which represents Br, and which represents Br-?
615
views
 Verified step by step guidance
Verified step by step guidance

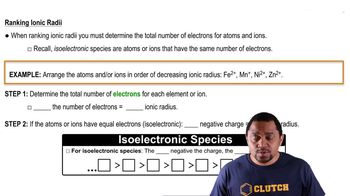

Which of these spheres represents F, which represents Br, and which represents Br-?
In the following reaction
which sphere represents a metal and which represents a nonmetal?
Shown below is a qualitative diagram of the atomic orbital energies for an Na atom. The number of orbitals in each subshell is not shown.
(d) A sodium vapor lamp (Figure 7.23) operates by using electricity to excite the highest-energy electron to the next highest-energy level. Light is produced when the excited electron drops back to the lower level. Which two energy levels are involved in this process for the Na atom?