A 1.0-g sample of carbon dioxide (CO2) is fully decomposed into its elements, yielding 0.273 g of carbon and 0.727 g of oxygen. (b) If a sample of a different compound decomposes into 0.429 g of carbon and 0.571 g of oxygen, what is its ratio of the mass of O to C?
Sodium reacts with oxygen in air to form two compounds: sodium oxide and sodium peroxide. In forming sodium oxide, 23.0 g of sodium combines with 8.0 g of hydrogen. In forming sodium peroxide, 23.0 g of sodium combines with 16.0 g of oxygen. (b) What fundamental law does this experiment demonstrate?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
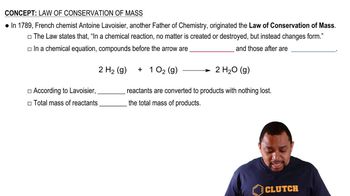
Law of Conservation of Mass
Law of Definite Proportions

Stoichiometry

A 1.0-g sample of carbon dioxide (CO2) is fully decomposed into its elements, yielding 0.273 g of carbon and 0.727 g of oxygen. If a sample of a different compound decomposes into 0.429 g of carbon and 0.571 g of oxygen, what is its ratio of the mass of O to C? (c) According to Dalton's atomic theory, what is the empirical formula of the second compound?
Sodium reacts with oxygen in air to form two compounds: sodium oxide and sodium peroxide. In forming sodium oxide, 23.0 g of sodium combines with 8.0 g of hydrogen. In forming sodium peroxide, 23.0 g of sodium combines with 16.0 g of oxygen. (a) What are the mass ratios of oxygen in the two compounds?
A chemist finds that 30.82 g of nitrogen will react with 17.60, 35.20, 70.40, or 88.00 g of oxygen to form four different compounds. (b) How do the numbers in part (a) support Dalton's atomic theory?
In a series of experiments, a chemist prepared three different compounds that contain only iodine and fluorine and determined the mass of each element in each compound: Compound Mass of Iodine (g) Mass of Fluorine (g) 1 4.75 3.56 2 7.64 3.43 3 9.41 9.86 (b) How do the numbers in part (a) support the atomic theory?
