The potassium-ion concentration in blood plasma is about 5.0⨉10-3 M, whereas the concentration in muscle-cell fluid is much greater (0.15 M ). The plasma and intracellular fluid are separated by the cell membrane, which we assume is permeable only to K+. (a) What is ΔG for the transfer of 1 mol of K+ from blood plasma to the cellular fluid at body temperature 37 °C? (b) What is the minimum amount of work that must be used to transfer this K+?
Ch.19 - Chemical Thermodynamics
Chapter 19, Problem 107
An ice cube with a mass of 20 g at -20 °C (typical freezer temperature) is dropped into a cup that holds 500 mL of hot water, initially at 83 °C. What is the final temperature in the cup? The density of liquid water is 1.00 g>mL; the specific heat capacity of ice is 2.03 J>g@C; the specific heat capacity of liquid water is 4.184 J>g@C; the enthalpy of fusion of water is 6.01 kJ>mol.

Verified Solution
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. It varies between different materials, influencing how they absorb and release heat. In this problem, the specific heat capacities of both ice and liquid water are crucial for calculating the heat transfer during the temperature change.
Recommended video:
Guided course

Heat Capacity
Enthalpy of Fusion
The enthalpy of fusion is the amount of energy required to change a substance from solid to liquid at its melting point without changing its temperature. For water, this value is significant when ice melts into liquid water, as it requires energy input, which affects the overall heat balance in the system.
Recommended video:
Guided course

Enthalpy of Formation
Heat Transfer and Equilibrium
Heat transfer refers to the movement of thermal energy from a hotter object to a cooler one until thermal equilibrium is reached. In this scenario, the heat lost by the hot water will be equal to the heat gained by the ice, including the energy needed for melting. Understanding this principle is essential for determining the final temperature of the system.
Recommended video:
Guided course
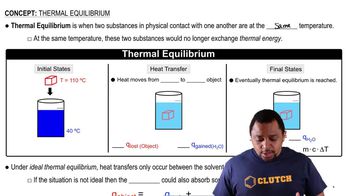
Thermal Equilibrium
Related Practice
Textbook Question
1097
views
1
rank
Textbook Question
In chemical kinetics, the entropy of activation is the entropy
change for the process in which the reactants reach the
activated complex. Predict whether the entropy of activation
for a bimolecular process is usually positive or
negative.
1493
views
Textbook Question
At what temperatures is the following reaction, the reduction of magnetite by graphite to elemental iron, spontaneous? Fe3O4(s) + 2 C(s, graphite) → 2 CO2(g) + 3 Fe(s)
795
views
1
comments
Textbook Question
Carbon disulfide 1CS22 is a toxic, highly flammable substance. The following thermodynamic data are available for CS21l2 and CS21g2 at 298 K:
