The solubility of CaCO3 is pH dependent. (b) Use the Kb expression for the CO32 - ion to determine the equilibrium constant for the reaction CaCO31s2 + H2O1l2 ΔCa2 + 1aq2 + HCO3-1aq2 + OH-1aq2
Calculate the solubility of Mg1OH22 in 0.50 M NH4Cl.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
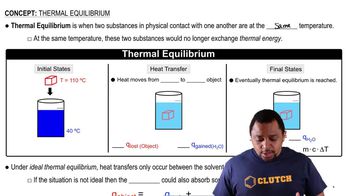
Key Concepts
Solubility Product Constant (Ksp)

Common Ion Effect

Ionic Equilibrium
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca51PO423OH, and whose corresponding Ksp = 6.8 * 10-27. As discussed in the Chemistry and Life box on page 746, fluoride in fluorinated water or in toothpaste reacts with hydroxyapatite to form fluoroapatite, Ca51PO423F, whose Ksp = 1.0 * 10-60. (a) Write the expression for the solubility-constant for hydroxyapatite and for fluoroapatite.
The solubility-product constant for barium permanganate, Ba1MnO422, is 2.5 * 10-10. Assume that solid Ba1MnO422 is in equilibrium with a solution of KMnO4. What concentration of KMnO4 is required to establish a concentration of 2.0 * 10-8 M for the Ba2 + ion in solution?
The solubility product constants of PbSO4 and SrSO4 are 6.3 * 10-7 and 3.2 * 10-7, respectively. What are the values of 3SO4 2 - 4, 3Pb2 + 4, and 3Sr2 + 4 in a solution at equilibrium with both substances?
