Textbook Question
The average pH of normal arterial blood is 7.40. At normal body temperature 137 °C2, Kw = 2.4 * 10-14. Calculate 3H+4, 3OH-4, and pOH for blood at this temperature.
2149
views
1
rank
 Verified step by step guidance
Verified step by step guidance


The average pH of normal arterial blood is 7.40. At normal body temperature 137 °C2, Kw = 2.4 * 10-14. Calculate 3H+4, 3OH-4, and pOH for blood at this temperature.
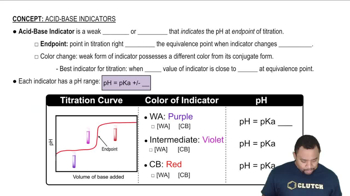
Addition of the indicator methyl orange to an unknown solution leads to a yellow color. The addition of bromthymol blue to the same solution also leads to a yellow color. (b) What is the range (in whole numbers) of possible pH values for the solution?
Addition of phenolphthalein to an unknown colorless solution does not cause a color change. The addition of bromthymol blue to the same solution leads to a yellow color. (c) What other indicator or indicators would you want to use to determine the pH of the solution more precisely?