Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (c) decrease [O2]
Ch.15 - Chemical Equilibrium
Chapter 15, Problem 62f
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (f) increase temperature.
 Verified step by step guidance
Verified step by step guidance1
Identify the type of reaction: The given reaction is exothermic, as indicated by the negative \( \Delta H = -904.4 \) kJ.
Apply Le Chatelier's Principle: In an exothermic reaction, increasing the temperature will shift the equilibrium position to favor the endothermic direction, which is the reverse reaction in this case.
Determine the effect on NO yield: Since the equilibrium shifts towards the reactants (reverse reaction), the yield of NO will decrease.
Consider the overall impact: Increasing temperature in an exothermic reaction generally decreases the yield of products formed in the forward reaction.
Conclude: Therefore, increasing the temperature will decrease the yield of NO at equilibrium.

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Le Chatelier's Principle
Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change. In the context of temperature changes, if the reaction is exothermic (releases heat), increasing the temperature will shift the equilibrium to favor the reactants, thereby decreasing the yield of products.
Recommended video:
Guided course

Le Chatelier's Principle
Exothermic Reactions
An exothermic reaction is one that releases energy in the form of heat. In the given reaction, the negative ΔH value indicates that it is exothermic. When the temperature is increased, the system will respond by favoring the endothermic direction (the reverse reaction) to absorb the added heat, which reduces the production of NO.
Recommended video:
Guided course
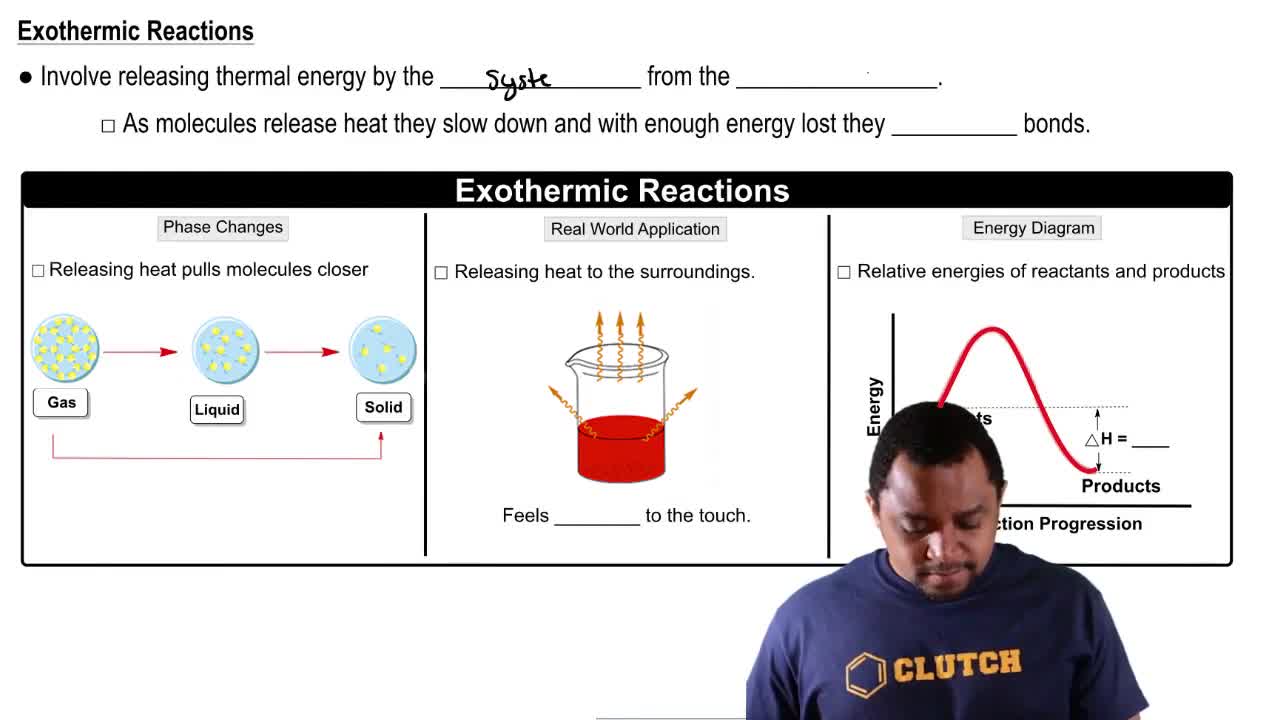
Endothermic & Exothermic Reactions
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium for a given reaction at a specific temperature. Changes in temperature can alter the value of K, affecting the position of equilibrium. For exothermic reactions, increasing temperature decreases K, leading to a lower concentration of products like NO at equilibrium.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
295
views
Textbook Question
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (d) decrease the volume of the container in which the reaction occurs
487
views
Textbook Question
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (e) add a catalyst
304
views
Open Question
How do the following changes affect the value of the equilibrium constant for a gas-phase exothermic reaction: (a) removal of a reactant, (b) removal of a product?
Open Question
For a certain gas-phase reaction, the fraction of products in an equilibrium mixture is increased by either increasing the temperature or by increasing the volume of the reaction vessel. Does the balanced chemical equation have more molecules on the reactant side or product side?
Textbook Question
Consider the following equilibrium between oxides of nitrogen 3 NO(g) ⇌ NO2(g) + N2O(g) (a) Use data in Appendix C to calculate ΔH° for this reaction.
469
views
