The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (b) What is the activation energy for the reverse reaction?
Indicate whether each statement is true or false. (b) Exothermic reactions are faster than endothermic reactions.

Verified Solution
Key Concepts
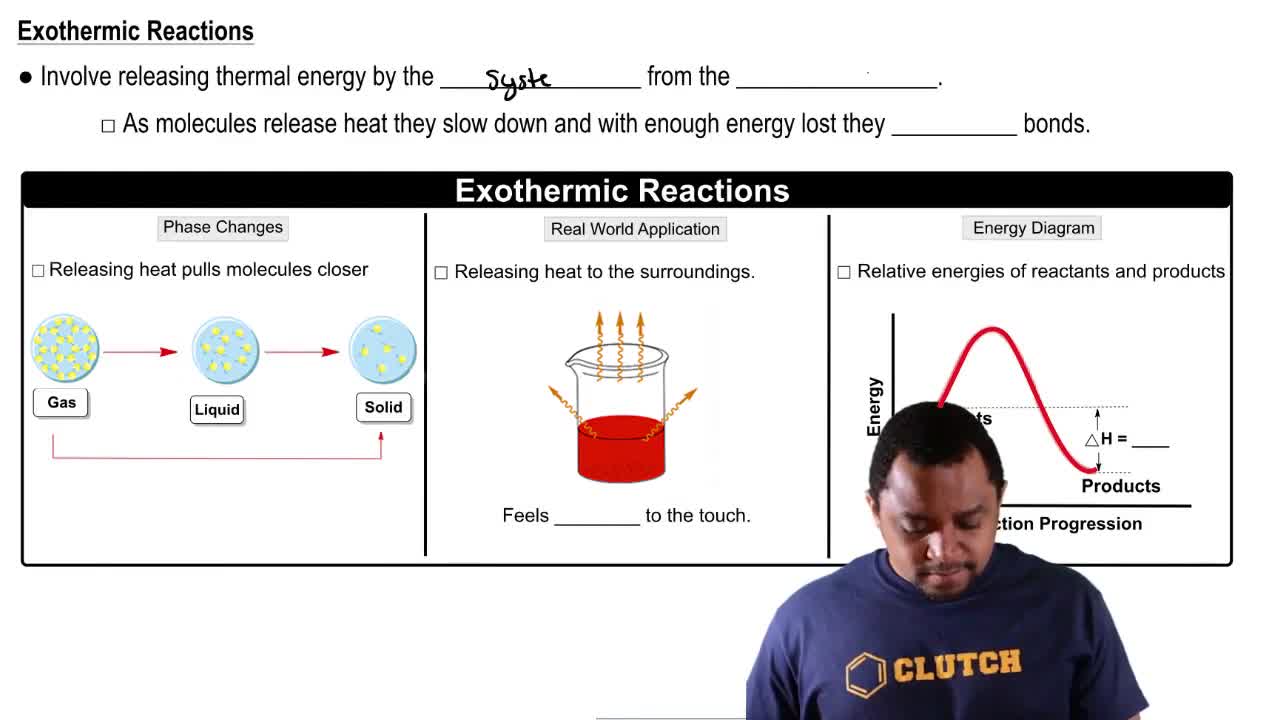
Exothermic Reactions
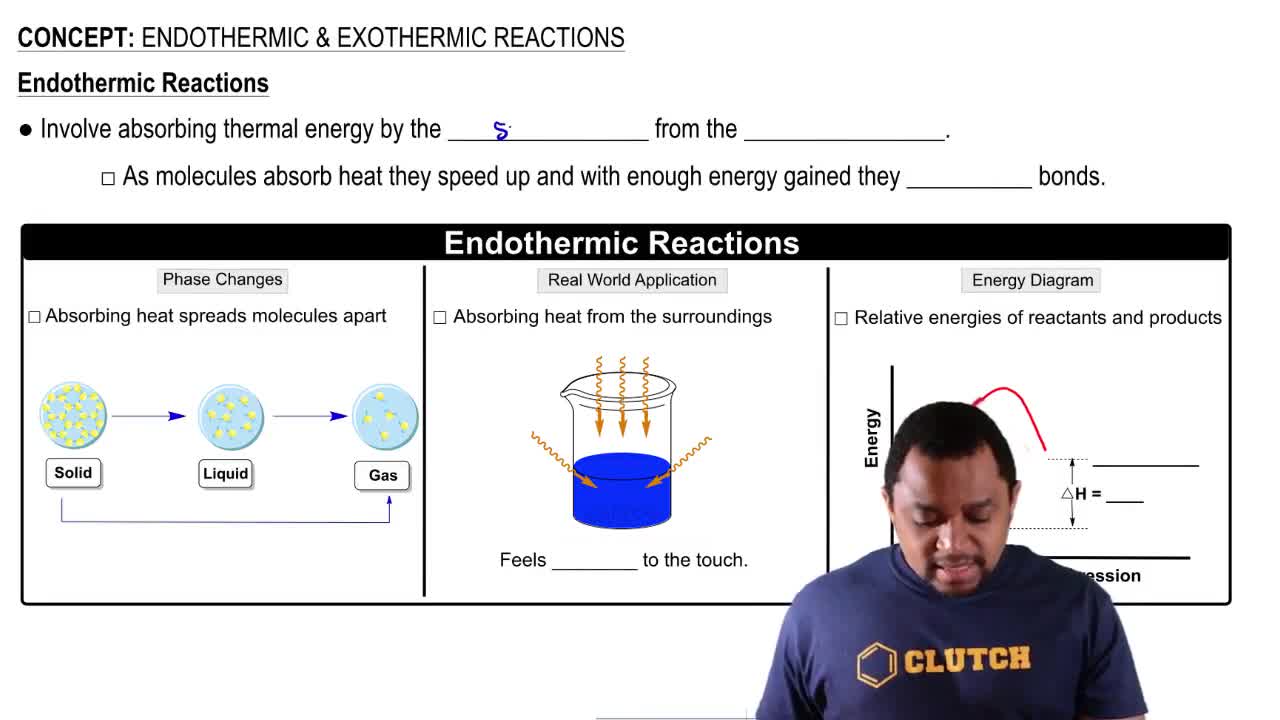
Endothermic Reactions
Reaction Rates

Indicate whether each statement is true or false. (c) Increasing the reaction temperature increases the fraction of successful collisions between reactants.
Indicate whether each statement is true or false. (a) If you measure the rate constant for a reaction at different temperatures, you can calculate the overall enthalpy change for the reaction.
Indicate whether each statement is true or false. (c) If you double the temperature for a reaction, you cut the activation energy in half.
Based on their activation energies and energy changes and assuming that all collision factors are the same, rank the following reactions from slowest to fastest. (a) Ea = 45 kJ>mol; E = -25 kJ>mol (b) Ea = 35 kJ>mol; E = -10 kJ>mol (c) Ea = 55 kJ>mol; E = 10 kJ>mol
The temperature dependence of the rate constant for a reaction is tabulated as follows: Temperature (K) k 1M 1 s1 2 600 0.028 650 0.22 700 1.3 750 6.0 800 23 Calculate Ea and A.
