Breathing air that contains 4.0 % by volume CO2 over time causes rapid breathing, throbbing headache, and nausea, among other symptoms. What is the concentration of CO2 in such air in terms of (a) mol percentage,

You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false. (a) The freezing point of the solution is unchanged by addition of the solvent.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Colligative Properties
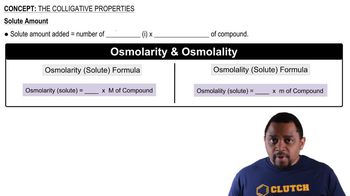
Freezing Point Depression

Nonvolatile Solute

Breathing air that contains 4.0 % by volume CO2 over time causes rapid breathing, throbbing headache, and nausea, among other symptoms. What is the concentration of CO2 in such air in terms of (b) molarity, assuming 1 atm pressure and a body temperature of 37 °C?
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false. (b) The solid that forms as the solution freezes is nearly pure solute.
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false. (d) The boiling point of the solution increases in proportion to the concentration of the solute. (e) At any temperature, the vapor pressure of the solvent over the solution is lower than what it would be for the pure solvent.
