The osmotic pressure of a 0.010 M aqueous solution of CaCl2 is found to be 0.674 atm at 25 °C. Calculate the van't Hoff factor, i, for the solution.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
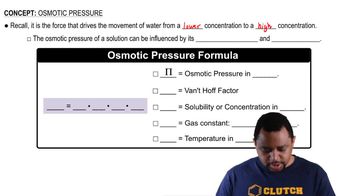
Osmotic Pressure
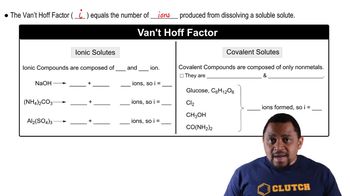
van't Hoff Factor (i)
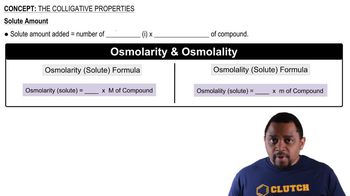
Colligative Properties
Lysozyme is an enzyme that breaks bacterial cell walls. A solution containing 0.150 g of this enzyme in 210 mL of solution has an osmotic pressure of 0.953 torr at 25 °C. What is the molar mass of lysozyme?
A dilute aqueous solution of an organic compound soluble in water is formed by dissolving 2.35 g of the compound in water to form 0.250 L of solution. The resulting solution has an osmotic pressure of 0.605 atm at 25 °C. Assuming that the organic compound is a nonelectrolyte, what is its molar mass?
Aerosols are important components of the atmosphere. Does the presence of aerosols in the atmosphere increase or decrease the amount of sunlight that arrives at the Earth's surface, compared to an 'aerosol-free' atmosphere? Explain your reasoning.
