Seawater contains 34 g of salts for every liter of solution. Assuming that the solute consists entirely of NaCl (in fact, over 90% of the salt is indeed NaCl), calculate the osmotic pressure of seawater at 20 °C
Ch.13 - Properties of Solutions
Chapter 13, Problem 81
Lysozyme is an enzyme that breaks bacterial cell walls. A solution containing 0.150 g of this enzyme in 210 mL of solution has an osmotic pressure of 0.953 torr at 25 °C. What is the molar mass of lysozyme?
 Verified step by step guidance
Verified step by step guidance1
Identify the formula for osmotic pressure: \( \Pi = iMRT \), where \( \Pi \) is the osmotic pressure, \( i \) is the van't Hoff factor (which is 1 for non-electrolytes like lysozyme), \( M \) is the molarity, \( R \) is the ideal gas constant, and \( T \) is the temperature in Kelvin.
Convert the given osmotic pressure from torr to atm: \( 0.953 \text{ torr} \times \frac{1 \text{ atm}}{760 \text{ torr}} \).
Convert the temperature from Celsius to Kelvin: \( T = 25 + 273.15 \).
Rearrange the osmotic pressure formula to solve for molarity \( M \): \( M = \frac{\Pi}{RT} \).
Calculate the molar mass of lysozyme using the formula: \( \text{Molar mass} = \frac{\text{mass of solute (g)}}{\text{moles of solute}} \), where moles of solute can be found from \( M \times \text{volume of solution in liters} \).

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane. It is directly related to the concentration of solute particles in the solution and can be calculated using the formula π = iCRT, where π is the osmotic pressure, i is the van 't Hoff factor, C is the molarity of the solution, R is the ideal gas constant, and T is the temperature in Kelvin.
Recommended video:
Guided course
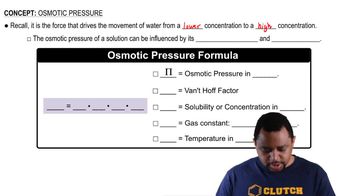
Osmotic Pressure Formula
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It can be determined by dividing the mass of the substance by the number of moles present. In the context of the question, calculating the molar mass of lysozyme involves using the osmotic pressure to find the number of moles in the solution and then relating that to the mass of the enzyme.
Recommended video:
Guided course

Molar Mass Concept
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. Although it primarily applies to gases, it can be adapted for solutions in terms of osmotic pressure. Understanding this law is essential for converting osmotic pressure into molarity, which is necessary for calculating the molar mass of lysozyme in the given scenario.
Recommended video:
Guided course

Ideal Gas Law Formula
Related Practice
Textbook Question
832
views
Textbook Question
Adrenaline is the hormone that triggers the release of extra glucose molecules in times of stress or emergency. A solution of 0.64 g of adrenaline in 36.0 g of CCl4 elevates the boiling point by 0.49 °C. Calculate the approximate molar mass of adrenaline from this data.
844
views
Textbook Question
Lauryl alcohol is obtained from coconut oil and is used to
make detergents. A solution of 5.00 g of lauryl alcohol in
0.100 kg of benzene freezes at 4.1 °C. What is the molar
mass of lauryl alcohol from this data?
2267
views
Textbook Question
A dilute aqueous solution of an organic compound soluble in water is formed by dissolving 2.35 g of the compound in water to form 0.250 L of solution. The resulting solution has an osmotic pressure of 0.605 atm at 25 °C. Assuming that the organic compound is a nonelectrolyte, what is its molar mass?
1035
views
Textbook Question
The osmotic pressure of a 0.010 M aqueous solution of CaCl2 is found to be 0.674 atm at 25 °C. Calculate the van't Hoff factor, i, for the solution.
2344
views
1
rank
Open Question
Based on the data given in Table 13.4, which solution would give the larger freezing-point lowering, a 0.030 m solution of NaCl or a 0.020 m solution of K2SO4?
