Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
436
views
 Verified step by step guidance
Verified step by step guidance

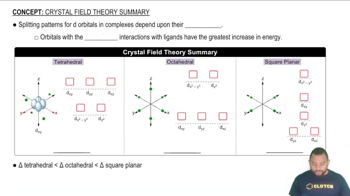

By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (c) Ce2(SO4)3.
Which of the following in each pair is likely to be more soluble in hexane, C6H14: b. benzene (C6H6) or glycerol, CH2(OH)CH(OH)CH2OH,
Which of the following in each pair is likely to be more soluble in water: (a) cyclohexane (C6H12) or glucose (C6H12O6),