The 'free-base' form of cocaine (C17H21NO4) and its protonated hydrochloride form (C17H21NO4) are shown below; the free-base form can be converted to the hydrochloride form with one equivalent of HCl. For clarity, not all the carbon and hydrogen atoms are shown; each vertex represents a carbon atom with the appropriate number of hydrogen atoms so that each carbon makes four bonds to other atoms (e) How many mL of a concentrated 18.0 M HCl aqueous solution would it take to convert 1.00 kilograms (a 'kilo') of the free-base form of cocaine into its hydrochloride form?
Most fish need at least 4 ppm dissolved O2 in water for survival. (b) What partial pressure of O2 above water is needed to obtain 4 ppm O2 in water at 10 °C? (The Henry's law constant for O2 at this temperature is 1.71⨉10-3 mol/L-atm.)

Verified Solution
Key Concepts
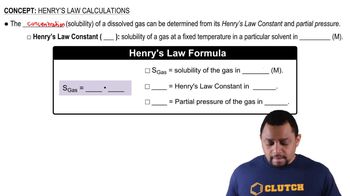
Henry's Law
Dissolved Oxygen Concentration

Temperature Effects on Gas Solubility

A supersaturated solution of sucrose (C12H22O11) is made by dissolving sucrose in hot water and slowly letting the solution cool to room temperature. After a long time, the excess sucrose crystallizes out of the solution. Indicate whether each of the following statements is true or false: (b) After the excess sucrose has crystallized out, the system is now unstable and is not in equilibrium.
Most fish need at least 4 ppm dissolved O2 in water for survival. (a) What is this concentration in mol/L?
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (a) Assuming that the solubility of radon in water with 1 atm pressure of the gas over the water at 30 °C is 7.27⨉10-3 M, what is the Henry's law constant for radon in water at this temperature?
The maximum allowable concentration of lead in drinking water is 9.0 ppb. (b) How many grams of lead are in a swimming pool containing 9.0 ppb lead in 60 m3 of water?
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the concentration of the solution in (a) molality,
