Here are the essential concepts you must grasp in order to answer the question correctly.
Liquid Crystals
Liquid crystals are materials that exhibit properties between those of conventional liquids and solid crystals. They can flow like a liquid but have some degree of molecular order, which allows them to respond to electric and magnetic fields. Understanding the different phases of liquid crystals, such as nematic and smectic, is crucial for applications in displays and other technologies.
Recommended video:
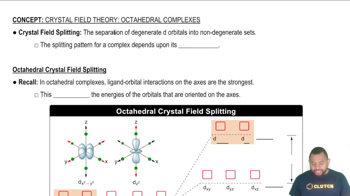
The crystal field splitting pattern for octahedral complexes has the d orbitals on or along the axes as having the higher energy.
Nematic Phase
The nematic phase is a type of liquid crystal phase where the molecules are oriented in the same direction but do not have positional order. This means that while the long axes of the molecules are aligned, they can move freely, leading to fluid-like behavior. The lack of positional order distinguishes it from more ordered phases, such as smectic.
Recommended video:
Phase Changes in Diagrams
Smectic Phase
The smectic phase is characterized by a higher degree of order compared to the nematic phase. In this phase, molecules are not only aligned in the same direction but also organized into layers, with positional order within each layer. This layered structure contributes to unique physical properties, such as increased viscosity and different optical characteristics, making it more ordered than the nematic phase.
Recommended video:
Phase Changes in Diagrams