What molecular structural features cause high-density polyethylene to be denser than low-density polyethylene?
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (b) Would appropriately sized CdS quantum dots be able to emit blue light?

Verified Solution
Key Concepts
Band Gap Energy

Quantum Dots
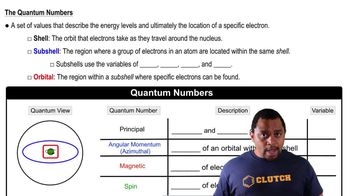
Photoluminescence
Indicate whether each statement is true or false: (a) Elastomers are rubbery solids.
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (a) What color is the emitted light?
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (c) What about red light?
Which statement correctly describes a difference between graphene and graphite? (a) Graphene is a molecule but graphite is not. (b) Graphene is a single sheet of carbon atoms and graphite contains many, and larger, sheets of carbon atoms. (c) Graphene is an insulator but graphite is a metal. (d) Graphite is pure carbon but graphene is not. (e) The carbons are sp2 hybridized in graphene but sp3 hybridized in graphite.
Selected chlorides have the following melting points: NaCl (801 °C), MgCl2 (714 °C), PCl3 (-94 °C), SCl2 (-121 °C) (a) For each compound, indicate what type its solid form is (molecular, metallic, ionic, or covalent-network).
