(a) Place the following substances in order of increasing volatility: CH4, CBr4, CH2Cl2, CH3Cl, CHBr3, and CH2Br2. (b) How do the boiling points vary through this series? (c) Explain your answer to part (b) in terms of intermolecular forces.
Ch.11 - Liquids and Intermolecular Forces
Chapter 11, Problem 54
You are high up in the mountains and boil water to make some tea. However, when you drink your tea, it is not as hot as it should be. You try again and again, but the water is just not hot enough to make a hot cup of tea. Which is the best explanation for this result? (a) High in the mountains, it is probably very dry, and so the water is rapidly evaporating from your cup and cooling it. (b) High in the mountains, it is probably very windy, and so the water is rapidly evaporating from your cup and cooling it. (c) High in the mountains, the air pressure is significantly less than 1 atm, so the boiling point of water is much lower than at sea level. (d) High in the mountains, the air pressure is significantly less than 1 atm, so the boiling point of water is much higher than at sea level.
 Verified step by step guidance
Verified step by step guidance1
Identify the problem: The tea is not as hot as expected when boiled at high altitudes.
Recall that boiling point is affected by atmospheric pressure. At higher altitudes, atmospheric pressure is lower than at sea level.
Understand that a lower atmospheric pressure results in a lower boiling point for water. This means water boils at a temperature less than 100°C.
Consider the options: (a) and (b) suggest evaporation due to dryness or wind, which are not the primary reasons for the lower temperature of boiling water. (d) incorrectly states that boiling point is higher at lower pressures.
Conclude that the correct explanation is (c): High in the mountains, the air pressure is significantly less than 1 atm, so the boiling point of water is much lower than at sea level.
Recommended similar problem, with video answer:

Verified Solution
This video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Boiling Point and Atmospheric Pressure
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. At higher altitudes, such as in the mountains, the atmospheric pressure is lower than at sea level, which decreases the boiling point of water. This means that water will boil at a lower temperature, resulting in less heat available for making hot tea.
Recommended video:
Guided course

Boiling Point Elevation
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid form. As temperature increases, the vapor pressure of a liquid also increases, leading to boiling. In high-altitude conditions, the lower atmospheric pressure allows water to reach its boiling point at a lower temperature, which can affect the temperature of the water used for brewing tea.
Recommended video:
Guided course

Raoult's Law and Vapor Pressure
Heat Transfer and Evaporation
Heat transfer occurs when thermal energy moves from a hotter object to a cooler one. Evaporation is a cooling process where molecules at the surface of a liquid gain enough energy to escape into the vapor phase. In high-altitude environments, rapid evaporation can occur, which may further cool the remaining liquid, making it difficult to achieve the desired temperature for hot tea.
Recommended video:
Guided course
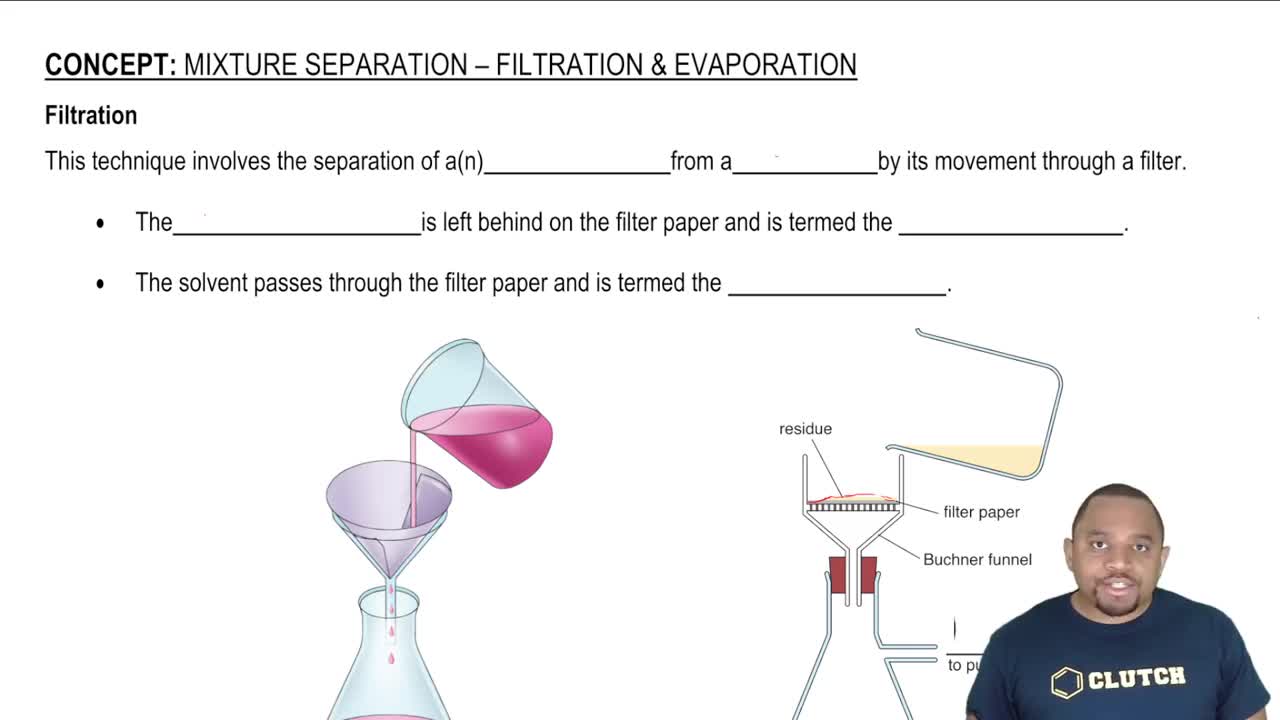
Filtration and Evaporation
Related Practice
Textbook Question
3117
views
1
rank
Open Question
True or false: (a) CBr4 is more volatile than CCl4. (b) CBr4 has a higher vapor pressure at the same temperature than CCl4.
Textbook Question
(a) Two pans of water are on different burners of a stove. One pan of water is boiling vigorously, while the other is boiling gently. What can be said about the temperature of the water in the two pans?
662
views
Textbook Question
Using the vapor-pressure curves in Figure 11.25, (d) estimate the external pressure at which diethyl ether will boil at 40 °C.
1150
views
Textbook Question
Appendix B lists the vapor pressure of water at various external pressures. (c) A city at an altitude of 5000 ft above sea level has a barometric pressure of 633 torr. To what temperature would you have to heat water to boil it in this city?
759
views
Open Question
What is the significance of the critical point in a phase diagram? Why does the line that separates the gas and liquid phases end at the critical point?
