Here are the essential concepts you must grasp in order to answer the question correctly.
Mole Fraction
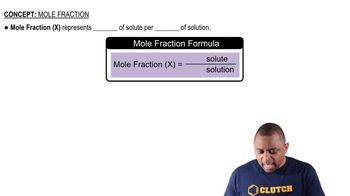
Mole fraction is a way of expressing the concentration of a component in a mixture. It is defined as the ratio of the number of moles of a specific component to the total number of moles of all components in the mixture. This dimensionless quantity helps in understanding the composition of gas mixtures and is crucial for calculations involving partial pressures and gas laws.
Recommended video:
Calculating Moles from Mass
To find the mole fraction, one must first convert the mass of each gas into moles using the formula: moles = mass (g) / molar mass (g/mol). The molar mass is a characteristic property of each substance, allowing for the determination of how many moles are present in a given mass. This step is essential for accurately calculating the mole fractions of the gases in the mixture.
Recommended video:
Molar Mass Calculation Example
Total Moles in a Mixture
The total number of moles in a mixture is the sum of the moles of all individual components. Once the moles of each gas are calculated, they are added together to find the total moles. This total is necessary for determining the mole fraction of each component, as it serves as the denominator in the mole fraction formula, ensuring that the fractions represent the correct proportions of each gas in the mixture.
Recommended video: