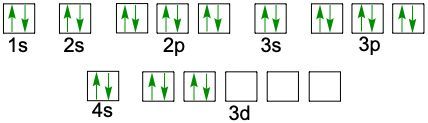
So here, if we reimagine the periodic table, we'll see it in this depiction. Now realize here we have blue, we have yellow, we have purple, and we have red sectors. Now each of these is called a block. The S block is what's in blue. This is the P block, the D block, and the F block.
Remember, the S sublevel has one orbital and that one orbital can hold a maximum of two electrons, which is why the S block is these two columns to represent the two maximum electrons that the S sublevel can hold. The P sublevel has three orbitals, right? And each one again can hold a maximum of two electrons. So P theoretically can hold a maximum of 6 electrons. That's why the P block has 12345 and six slots for it.
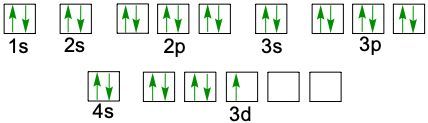
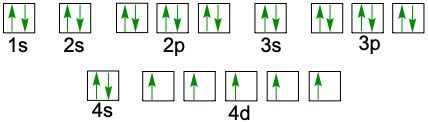
D can have up to 10 electrons because it has five orbitals. And if you count you'll see in here there's ten spots. And then for the F sublevels they can hold U to 14 electrons. And if you were to count these rows in red, you'd see they come out to 14. Now realize here that in this periodic table, the first slide here, which represents hydrogen, starts off our electron configuration as 1S1. Then as we move to the next one we add another electrons. So Helium is 1S2.
Then when we get to the second row, since we're in the 2nd row, we now have two. This is still the block, so this is 1S22S1 and it continues onward and onward. Over here we'd go 1S22S22P1. So following this pattern this would be 3S44S55S66S7 and then here this would be 3P44P55P66P7. When we go to the D block, realize that it's going to drop down by 1, so here this is 4S3. But then when we go to D block it drops down by 1 number so now it's 3D4. This would be 4D55D6.
Now notice how this number goes from 57 to 72. That's because 58 to 71 are here. Remember this? This red line here says that this entire red row exists in between 57 and 72. And then we go between 89 and 104. Because 90 to 103 is down here. These are your F blocks. They also drop down by 1 number, so this is 4F5 and 5F6. So basically, if you can reimagine the periodic table in this fashion, you can use it to figure out the electron configuration of any element or ion given to you. So we're going to put this periodic table to use in order to do future electron configurations.