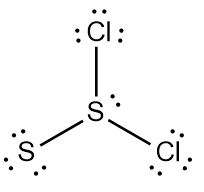
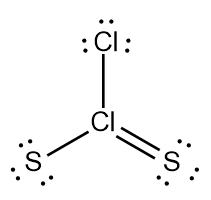
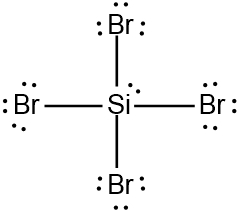
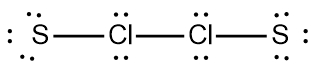So realize when it comes to drawing Lewis dot structures that there are many possibilities that exist, but these are the rules that we're going to follow in order to draw the best structure. Now recall elements form bonds in order to gain electrons and become like the noble gases, the nearest noble gas. If we take a look here at this example question, it says draw the Lewis dot structure for formaldehyde molecule which is CH2O.
All right, so in order to draw this let's look over the steps. So step one says we need to determine the total number of valence electrons of the structure. Recall valence electrons equal group number of the element. So here we have carbon which has four valence electrons +2 hydrogens. Each one has one itself. That's a total of 2 valence electrons. Oxygens in Group 6A, so it has six valence electrons. Adding all that up together gives us 12 total valence electrons.
Now Step 2, we're going to place the least electronegative element in the center and connect all elements with single bonds. All right. Now, there are exceptions to this exceptions. Hydrogen can never go in the center. Recall hydrogen only needs one more valence electron to adopt the same type of numbers of valence electrons as helium. So it does. It can't be in the center, it'd be overflowing with electrons. Also halogens. So remember the elements in groups 7A, Salt, fluorine, chlorine, bromine and iodine? They only make single bonds as a surrounding element.
All right, So what we're going to do here is we're going to put into practical We just set so we can't put hydrogen in the center. So our next best option is carbon. So carbon goes in the center and then we're going to place hydrogen and oxygen just to keep it like symmetrical. I put the hydrogens on the sides and the oxygen at the top. We said we're going to connect all of these elements with single bonds. So we're going to connect single, single and single.
Now add electrons to all surrounding elements until they have 8 electrons following the octet rule. So we want them to have 8 octet electrons around them. Exceptions. Hydrogens only want 2 octet electrons around them, because again, when they get to 2 octet electrons, they behave just like helium, their nearest noble gas. All right, so oxygen, we needed to have 8 octet electrons by following the octet rule. So right now we have these two electrons, so 3, 4, 5, 6, 7 and 8. And actually, let me bring that down so we get a better look at it.
So carbon goes here, and then we're connecting the hydrogens and we're connecting the oxygen. And then we have again these two electrons that are within this bond. Oxygen needs 8, so 3, 4, 5, 6, 7 and 8 right Now place any remaining electrons on the central element. So realize how many electrons have we used so far. We have 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, so we have 12 electrons already used, so we don't have any additional electrons left to put around the carbon atom.
But this is what's important for Step 5. If any elements don't have 8 octet electrons, add double or triple bonds between them. If we look, carbon has 2, 4, 6 electrons around it. It needs two more to get to the octet rule. So what are we going to do? We're going to use one of these pair of electrons and bring it down and when we do that. So I'm going to take these pair of electrons and I'm going to bring them down and make a double bond with the carbon. So now we have a double bond with that carbon, and now carbon has a total of 8 electrons around itself. So what's following the octet rule? And the oxygen up above is also following the octet rule.
Now step 6. The formal charge can be used to determine if a Lewis dot structure is drawn correctly. The only allowable formal charges for an element are -1, zero, or +1. For stability, draw the structure that gives least formal least formal charges or lowest formal charges, and realize you're going to put the negative formal charge on the more electronegative element because that tends to be the most stable. In this case we don't have to do that because we see that the oxygen is following the octet rule by having 8 total octet electrons around it. Same with carbon. Hydrogen only makes single bonds, so we see that they're both making single bonds so we know they're satisfied as well.
So that means for the formaldehyde molecule we'd have hydrogen single bonded and then the oxygen and carbon would be double bonded to one another and then the oxygen would have these four non bonding electrons around itself. So this would be the best structure for formaldehyde.