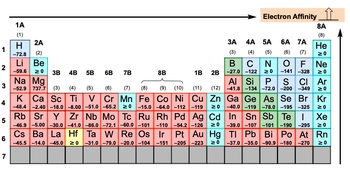
Here we're saying that electron affinity, abbreviated as EA, is the energy released, making it exothermic from the addition of an electron to a gaseous atom, or ion in kilojoules. Now you may also get it in kilojoules per mole, but for the most part we're going to say it's in just in kilojoules. Now we're going to say here we're talking about adding an electron to an atom or an ion.
So here we have neutral carbon, we're going to add an electron to it. So here is the electron we're adding. When it gains that electron, it becomes more negative. So now it's going to be C- as a gas. Now here this is an exothermic reaction. So in an exothermic reaction, we release energy in order to create a bond. Releasing energy means that we're going to have a negative sign for electron affinity.
Now we're going to say here that the more negative the electron affinity value is, then the more exothermic the reaction becomes. And we're going to say here that there are exceptions. When we look at the periodic table in the next video, there are exceptions to electron affinity. For the exceptions, we're going to say that they are equal to or greater than 0, and that means the element will not readily accept an electron.
So if your electron affinity is 0 or higher, that means that element does not want to accept or it cannot accommodate an electron because it interrupts their stability. So now that we've talked about electron affinity, click on to the next video and let's take a look at the periodic table.