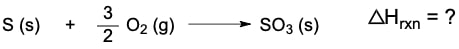
Now Hesa's law has to do with the rearrangement of thermochemical equations to help us fund the overall enthalpy of reaction. Now recall a thermochemical equation is a chemical equation that involves or includes an enthalpy of reaction. Now we're going to say the thermochemical equation and its enthalpy of reaction, which is ΔHRXN are directly proportional. This means that any change to the original equation will cause the same change within your enthalpy of reaction.
So let's say we have an original equation here. Original thermochemical equation where we have two moles of magnesium is solid plus O2 gas and it produces 2 moles of magnesium oxide. The enthalpy associated with this is -1204 kilojoules. Now I can do different things, different rearrangements to this thermochemical equation that'll affect it, but also affect my delta H of reaction. So the things I can do is I can multiply this equation by a value, I can divide it by a number, or I can reverse the reaction.
So let's say we multiply it. Here we have our original equation, and let's say multiply it by two. Now if I multiply it by two, the coefficient's now become four, two and four. If I multiply the equation by two, then that means I also have to multiply my delta H by two. This original value of -1204 kilojoules gets multiplied by two, and now my new enthalpy of the reaction is -2408 kilojoules.
Now let's say instead of multiplying, I divided so my original equation again. Now I divide it by two. Dividing it by two changes my coefficients now to one for magnesium solid, to 1/2 for O2 and one for magnesium oxide. Again, whatever I do to the equation, I must do the same thing to my delta H. So -1204 gets divided by two so it becomes -602 kilojoules.
Finally, the last thing I can do to my thermochemical equation is I can reverse it. What was a product can become a reactant, so that's 2 MgO solid. And what was a reactant can now become products, so 2 Mg solid plus one O2. Now when I reverse the reaction, what that does is it reverses the sign of Delta H so it was a -1204. Now it's going to become a +1204. Just realize that if I reverse the reaction, I reverse the sign of Delta H. So just remember these are the three things I can do to a thermochemical equation and they have a direct impact on my Delta H of reaction for that particular thermochemical equation.