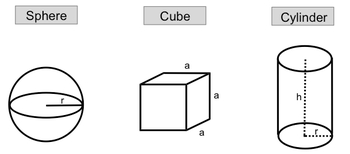
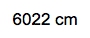So here in this example question, it says the density of silver is 10.5 grams per centimeters cubed. What is the mass in kilograms of a cube of silver that measures .56 meters on each side? All right, although we're talking about density here, and we're talking about a cube, we can still apply the rules that we learn when it comes to given amounts, conversion factors, and end amounts.
So in this question our end amount or given amount actually is the one that has only one unit connected to it and that would be our 0.56 meters. So this is our given amount. Then we have to think about where is our end amount, where do we need to go and where do we stop. Our end amount here would be kilograms, so we need kilograms from the silver cube. Next we look to see what kind of conversion factors are available. Here we have the density of the silver. Remember, density itself is a conversion factor because it's tying together two different units. So that would be 10.51 cm³.
All right, so now let's put it all together. So we have given here given amount. We have to get to end amount and remember to connect them together. We use our conversion factors. Now we see our conversion factor. There is one 10.5g per centimeters cubed. We can try to isolate these grams here. If I can isolate those grams, I can convert them later into kilograms. But to be able to isolate those grams I have to cancel out the centimeters cubed. Remember, centimeters cubed is just a unit for volume. Sometimes we're given an amount. We have our given amount, but sometimes we have to change it up. We have to change it so that we can use it within our dimensional analysis setup.
Since this whole section is on the density of geometric objects and we have a cube, I can determine the volume of the cube. Remember volume equals length cubed, so that would be 0.563 meters cubed, so that comes out to be 0.175616cm3. So this is my actual given amount that I'm going to utilize within this setup. I need to change it to meters cubed because from there I can convert it to centimeters cubed and then from centimeters cubed I'll be able to cancel out the centimeters from the density and be left with grams all right.
So our conversion factor one is just a metric prefix. Conversion meters go here on the bottom, centimeters go here on top. Remember the coefficient of one goes on the same side with the metric prefix SO1 centi is 10-2. Here these meters are cubed, but these are not. So I would have to cube the whole thing. Then we're going to have our meters. Cubes cancel out and now we have centimeters cubed. Because it's in centimeters cubed, I can now use my conversion factor too. So my conversion factor two. I bring this down 10.5g, 11 cm³ on the bottom centimeters cubes will cancel out and now I have grams of my substance, but I don't want it in grams, I need it in kilograms.
So one more conversion factor is actually another metric prefix conversion. So we're going to put here grams on the bottom, kilograms on top. Remember kilo is a metric prefix so 1 K is 103. So grams here cancel out and I'll be left with kilograms at the end. So when you plug this in initially it'll be 0.175616 times 10.5 / 10-2 which is going to be cubed divided by also 103. If you plug this incorrectly, you'll get 1843.968 kilograms. But looking back on the original question, .56 has two sig figs and 10.5 has three sig figs. So we have to go with the least number of significant figures. So getting this to two sick figs, we can write it either as 1800 kilograms or 1.8*103 kilograms. Either one is a reliable, reasonable answer.
So just remember sometimes our dimensional analysis set up the given amount has to be changed a little bit in order to fit and cancel out with other present units. By doing that, we're able to isolate our kilograms within this example. Now that we've done this question, let's move on to our practice question.