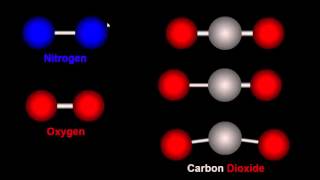
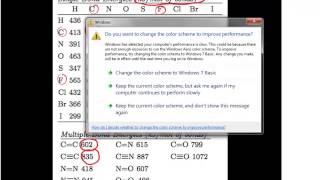
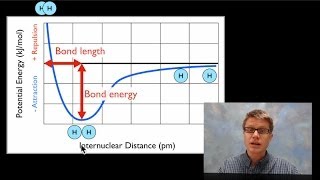
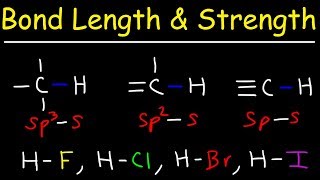11. Bonding & Molecular Structure
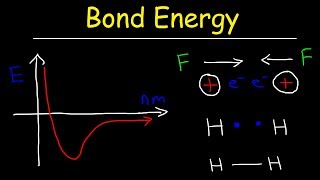
Bond Energy
11. Bonding & Molecular Structure
Bond Energy
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Consider the following equation:
Determine the bond enthalpy value for the F–S bond.
3296views4comments - Multiple Choice
Use the bond energies to estimate the enthalpy of reaction for the combustion of 5 moles of acetylene:
9087views7rank5comments - Multiple ChoiceCalculate the enthalpy of the following reaction based on average bond enthalpies.1053views
- Multiple ChoiceWhich of the following bonds is the strongest?1216views
- Open Question
Ethanol is a possible fuel. use average bond energies to calculate δHrxn for the combustion of ethanol. CH3CH2OH(g) + 3 O2(g) → 2 CO2(g) + 3 H2O(g)
921views - Open Question
Calculate the average molar bond enthalpy of the carbon–hydrogen bond in a CH4 molecule.
985views - Open Question
Use average bond energies to calculate δHrxn for the following hydrogenation reaction: H2C=CH2(g) + H2(g) → H3C−CH3(g)
1140views - Open Question
Calculate δhrxn for the combustion of octane (C8H18) by using average bond energies.
730views