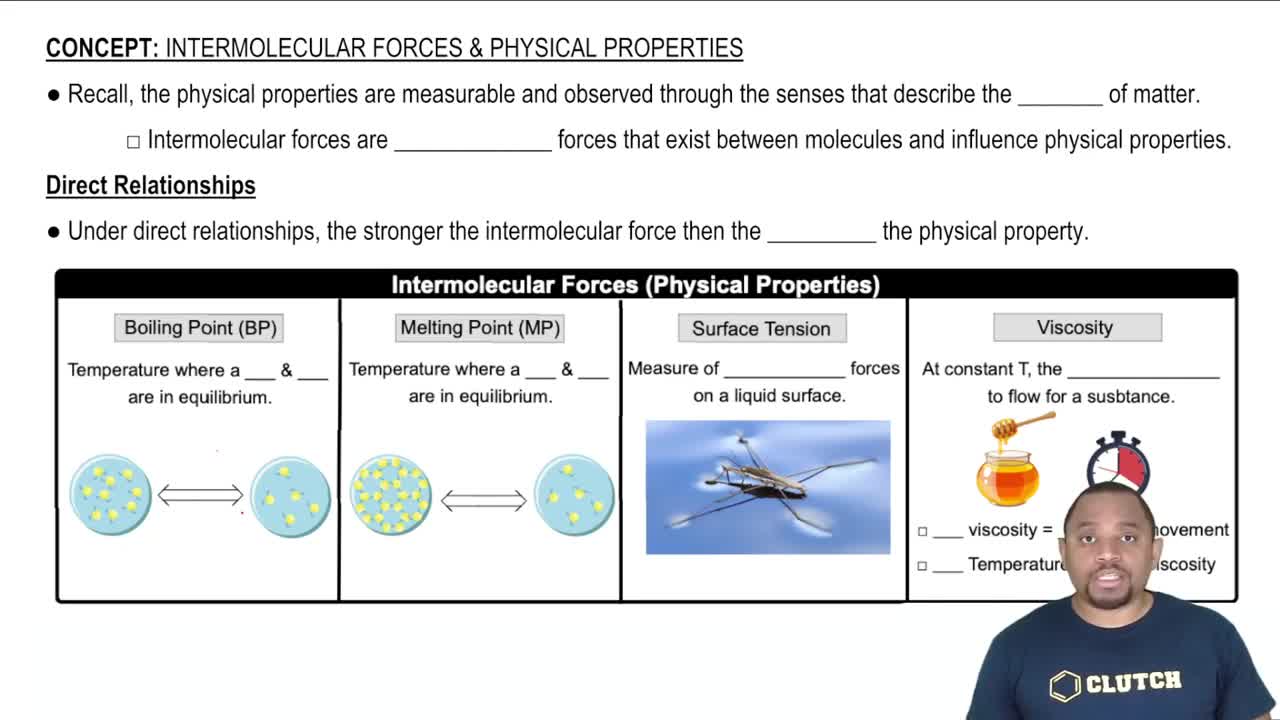
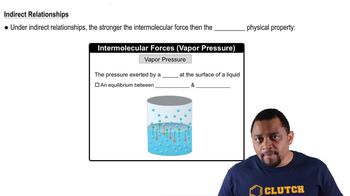
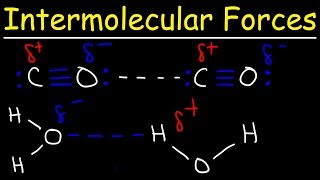
13. Liquids, Solids & Intermolecular Forces
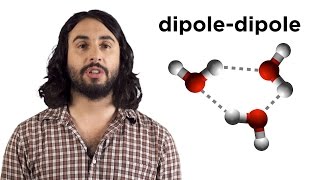
Intermolecular Forces and Physical Properties
13. Liquids, Solids & Intermolecular Forces
Intermolecular Forces and Physical Properties
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Which of the following will have the lowest boiling point?
2879views9rank1comments - Multiple Choice
Which molecules would most likely cause a liquid to have the lowest viscosity?
1768views11rank - Multiple ChoiceWhich of the following is a false statement about dispersion forces?1031views
- Multiple ChoiceWhich of the following statements is false about the changes occurring when ice is heated in an open beaker from −10.0℃ to water vapor at 110.0℃?751views
- Open Question
Ammonia and hydrogen fluoride both have unusually high boiling points due to _____.
1046views - Open Question
Which compound has the highest melting point: Al2(CO3)3, C12H22O11, C8H18, or H2O?
1177views - Open Question
A liquid with high viscosity _____ flow easily and _____ effective in wetting a surface.
908views - Open Question
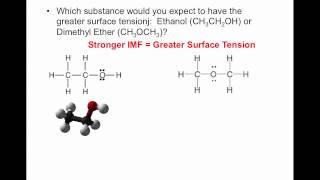
Which would have a higher vapor pressure: ethanol (C2H5OH) or dimethyl ether (CH3OCH3)?
1074views