Textbook Question
Name each alkane.
a. CH3-CH2-CH2-CH2-CH3
b.
100
views

 Verified step by step guidance
Verified step by step guidance
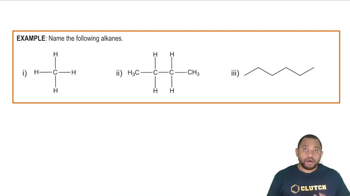


Name each alkane.
a. CH3-CH2-CH2-CH2-CH3
b.
Name each alkane.
c.
Name each alkane. d.
Draw a structure for each alkane. a. 3-ethylhexane c. 2,3-dimethylbutane
Draw a structure for each alkane.
b. 3-ethyl-3-methylpentane
Draw a structure for each alkane.
d. 4,7-diethyl-2,2-dimethylnonane