Textbook Question
List all the possible products for each alkane substitution reaction. (Assume monosubstitution.) b. CH3CH2CH3 + Cl2 → d.
431
views
 Verified step by step guidance
Verified step by step guidance


List all the possible products for each alkane substitution reaction. (Assume monosubstitution.) b. CH3CH2CH3 + Cl2 → d.
List all the possible products for each alkane substitution reaction. (Assume monosubstitution.) a.CH4 +Cl2 → b. CH3CH2Br + Br2 → d. CH3CHBr2 + Br2 →
Draw the correct structure for each compound. a. 2-hexene c. 4,4-dimethyl-2-hexene d. 3-ethyl-4-methyl-2-pentene
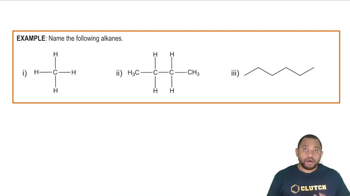
Name each alkane.
a. CH3-CH2-CH2-CH2-CH3
b.
Draw the correct structure for each compound. a. 4-octyne b. 3-nonene
Draw the structure for each compound. a. isopropylbenzene c. 1-chloro-4-methylbenzene