Give the numerical values of n and l corresponding to each of the following orbital designations: (d) 5d.
Ch.6 - Electronic Structure of Atoms
Chapter 6, Problem 59b
A certain orbital of the hydrogen atom has n = 4 and l = 3. (b) What are the possible values of ms for the orbital?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the quantum numbers. The principal quantum number (n) describes the energy level of the electron in the hydrogen atom. The azimuthal quantum number (l) describes the shape of the orbital. The magnetic quantum number (ml) describes the orientation of the orbital in space. The spin quantum number (ms) describes the spin of the electron.
Step 2: The spin quantum number (ms) has only two possible values, regardless of the values of the other quantum numbers. These values are +1/2 and -1/2.
Step 3: The +1/2 value represents an electron spinning in one direction (often referred to as 'spin up'), and the -1/2 value represents an electron spinning in the opposite direction (often referred to as 'spin down').
Step 4: Therefore, for any given orbital of the hydrogen atom, the possible values of ms are +1/2 and -1/2.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Quantum Numbers
Quantum numbers are a set of numerical values that describe the unique quantum state of an electron in an atom. The four quantum numbers are principal (n), azimuthal (l), magnetic (m_l), and spin (m_s). Each number provides specific information about the electron's energy level, shape, orientation, and spin direction.
Recommended video:
Guided course

Principal Quantum Number
Principal Quantum Number (n)
The principal quantum number (n) indicates the main energy level of an electron in an atom and can take positive integer values (1, 2, 3, ...). A higher value of n corresponds to a higher energy level and a greater average distance of the electron from the nucleus. In this case, n = 4 signifies that the electron is in the fourth energy level.
Recommended video:
Guided course

Principal Quantum Number
Spin Quantum Number (m_s)
The spin quantum number (m_s) describes the intrinsic angular momentum or 'spin' of an electron, which can have one of two possible values: +1/2 or -1/2. This property is crucial for determining the orientation of the electron's spin and is essential for understanding electron pairing and the Pauli exclusion principle in atomic structure.
Recommended video:
Guided course
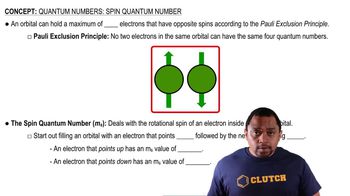
Spin Quantum Number
Related Practice
Textbook Question
791
views
Textbook Question
Give the values for n, l, and ml for (a) each orbital in the 3p subshell, (b) each orbital in the 4f subshell.
777
views
Textbook Question
A certain orbital of the hydrogen atom has n = 4 and l = 3. (a) What are the possible values of ml for this orbital?
891
views
Textbook Question
Which of the following represent impossible combinations of n and l? (a) 1p (b) 4s (c) 5f (d) 2d
1080
views
Textbook Question
For the table that follows, write which orbital goes with the quantum numbers. Don't worry about x, y, z subscripts. If the quantum numbers are not allowed, write 'not allowed.' n l ml Orbital 2 1 -1 2p (example) 1 0 0 3 -3 2 3 2 -2 2 0 -1 0 0 0 4 2 1 5 3 0
1056
views
Textbook Question
Sketch the shape and orientation of the following types of orbitals: (a) s, (b) pz, (c) dxy.
1177
views
