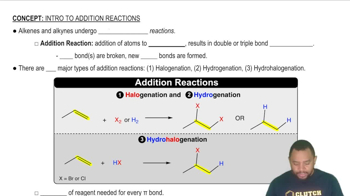
Here are the essential concepts you must grasp in order to answer the question correctly.
Alkene Structure
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond (C=C). This unsaturation makes them more reactive than alkanes, allowing them to undergo various addition reactions. The position and nature of substituents around the double bond can influence the products formed during these reactions.
Recommended video:
Addition Reactions
Addition reactions involve the addition of atoms or groups to the carbon atoms of the double bond in alkenes, resulting in the conversion of the double bond into a single bond. Common types of addition reactions include hydrogenation, halogenation, and hydrohalogenation, each producing different products based on the reagents used.
Recommended video:
Markovnikov's Rule
Markovnikov's Rule states that in the addition of HX (where X is a halogen) to an alkene, the hydrogen atom will attach to the carbon with the greater number of hydrogen atoms already attached. This rule helps predict the major product of the reaction, guiding the understanding of regioselectivity in alkene addition reactions.
Recommended video:
 Verified step by step guidance
Verified step by step guidance