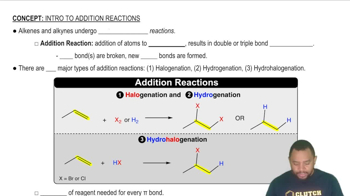
Here are the essential concepts you must grasp in order to answer the question correctly.
Alkene Structure
Alkenes are hydrocarbons that contain at least one carbon-carbon double bond (C=C). This unsaturation makes them more reactive than alkanes, allowing them to undergo various addition reactions. The geometry around the double bond is typically trigonal planar, which influences the orientation of the addition products.
Recommended video:
Addition Reactions
Addition reactions involve the addition of atoms or groups to the carbon atoms of the double bond in alkenes, resulting in the formation of saturated products. Common types of addition reactions include hydrogenation, halogenation, and hydrohalogenation, each producing different products based on the reagents used and the conditions of the reaction.
Recommended video:
Markovnikov's Rule
Markovnikov's Rule states that in the addition of HX (where X is a halogen) to an alkene, the hydrogen atom will attach to the carbon with the greater number of hydrogen atoms already attached. This rule helps predict the major product of the reaction, as it favors the formation of the more stable carbocation intermediate during the reaction process.
Recommended video: