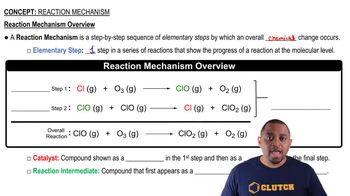
The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism:
H2O2(aq) + I-(aq) → H2O(l) + IO-(aq) (slow)
IO-(aq) + H2O2(aq) → H2O(l) + O2(g) + I-(aq) (fast)
(a) Write the chemical equation for the overall process.


![Graph showing the linear relationship of ln[H2] over time in chemical kinetics.](https://lightcat-files.s3.amazonaws.com/problem_images/b13cc8f7b3c6acff-1666285465032.jpg)