Identify at least two advantages of breathing air instead of water.
Explain how each parameter in Fick's law of diffusion is reflected in the structure of the mammalian lung.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Fick's Law of Diffusion

Alveolar Structure

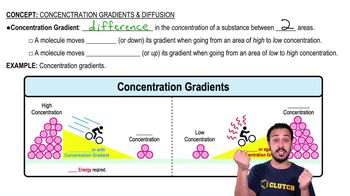
Concentration Gradient
Which of the following promotes oxygen release from hemoglobin? a. a decrease in temperature b. an increase in O2 level c. a decrease in pH d. a decrease in carbonic anhydrase activity
Describe the disadvantages of an open circulatory system relative to a closed circulatory system.
Frog lungs have a smaller surface area for gas exchange than mammalian lungs. How do frogs compensate for this difference? a. Frog tissue absorbs more oxygen from the blood than mammalian tissue does. b. Frogs breathe more quickly than mammals. c. Frogs also obtain oxygen via diffusion across the skin. d. Frog lung tissue has a greater density of capillary beds than mammalian lung tissue.
Carp are fishes that thrive in stagnant-water habitats with low oxygen partial pressure. Compared with the hemoglobin of many other fish species, carp hemoglobin has an extremely high affinity for O2. Draw an oxygen–hemoglobin equilibrium curve showing separate lines for carp and a fish that lives in water with a higher oxygen partial pressure. Explain why they differ.
Explain why a person who survives a myocardial infarction might need to have an artificial pacemaker implanted.
