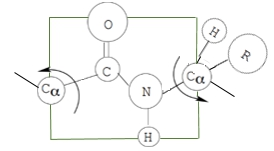
Understanding the peptide bond and the peptide group is essential for grasping the primary structure of proteins. The peptide bond itself is a specific type of amide covalent linkage that connects amino acids, forming the backbone of proteins. The peptide group encompasses not only the two atoms directly involved in the peptide bond but also their four neighboring atoms, totaling six atoms. These include the carbonyl group atoms, the amino group atoms, and the two adjacent alpha carbons.
In a dipeptide example, which consists of two amino acid residues, the unique characteristics of the peptide group can be observed. Each amino acid has a distinct R group; for instance, one may be glycine (with a hydrogen as its R group) and the other alanine (with a methyl group). The peptide bond connecting these residues is crucial for the protein's structure.
Resonance plays a significant role in the stability and characteristics of the peptide bond. The lone pair on the nitrogen can shift to the peptide bond, creating a resonance structure that shows a double bond character between the nitrogen and carbonyl carbon. This results in a dipole moment across the peptide bond, indicating that it is polarized, with electron density being unevenly distributed. The nitrogen carries a partial positive charge, while the carbonyl oxygen carries a partial negative charge, contributing to the bond's polarity.
To accurately represent the resonance, a hybrid resonance structure is often used. This structure illustrates the partial double bond character of the peptide bond and the carbonyl group, highlighting the importance of these features in protein structure and function. The polarization and resonance characteristics of the peptide bond are foundational concepts that will be further explored in subsequent lessons, emphasizing their significance in understanding protein behavior and interactions.