2. Cell Chemistry & Cell Components
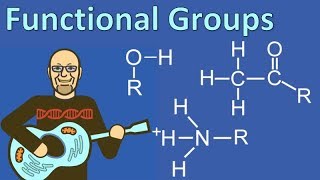
Functional Groups
2. Cell Chemistry & Cell Components
Functional Groups
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
What is the name of the functional group shown in the figure?
6565views80rank - Multiple Choice
All of the following are examples of functional groups in biology except:
5458views83rank1comments - Multiple ChoiceWhich of the following functional groups is present in all amino acids?2765views1rank
- Multiple ChoiceWhich of these is found in all amino acids?1996views
- Textbook QuestionWhat two functional groups are bound to the central carbon of every free amino acid monomer?a. an R-group and a hydroxyl groupb. an N—H group and a ═(C═O) groupc. an amino group and a hydroxyl groupd. an amino group and a carboxyl group2497views
- Textbook QuestionVISUAL SKILLS Which functional group is not present in this molecule? a. carboxylb. sulfhydrylc. hydroxyld. amino1493views
- Textbook QuestionMAKE CONNECTIONS Which chemical group is most likely to be responsible for an organic molecule behaving as a base?a. hydroxylb. carbonylc. aminod. phosphate1458views
- Textbook QuestionWhich of these functional groups is known to be used for storing large amounts of chemical energy?a. amino groupb. carbonyl groupc. phosphate groupd. sulfhydryl group1820views