Here are the essential concepts you must grasp in order to answer the question correctly.
First Law of Thermodynamics
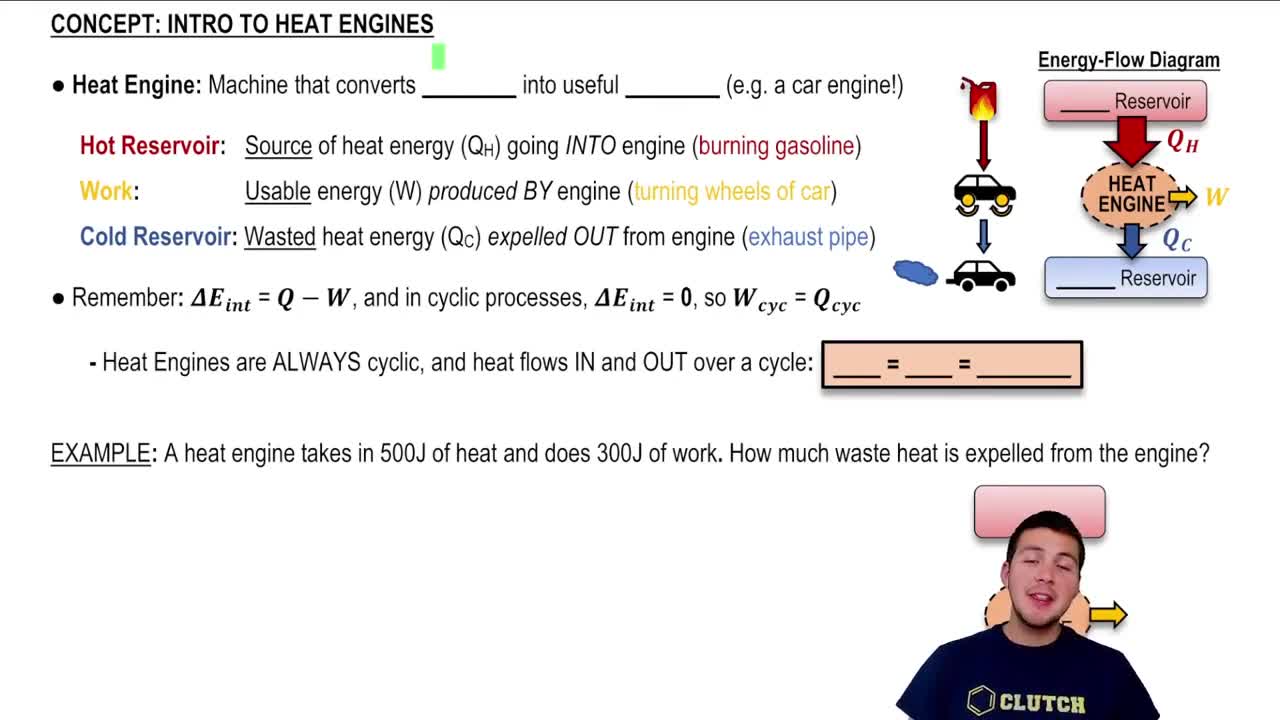
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another. In the context of a diesel engine, the work done by the engine and the heat exchanged must balance according to this principle. This law helps in understanding how the energy supplied to the engine is converted into mechanical work and heat.
Recommended video:
The First Law of Thermodynamics
Heat Transfer
Heat transfer refers to the movement of thermal energy from one object or system to another due to a temperature difference. In the case of the diesel engine, heat is supplied to the engine to facilitate combustion, and some of this energy is converted into work, while excess heat is discarded. Understanding heat transfer is crucial for calculating the total energy input required for the engine's operation.
Recommended video:
Overview of Heat Transfer
Efficiency of Heat Engines
The efficiency of a heat engine is defined as the ratio of the work output to the heat input, often expressed as a percentage. It indicates how effectively the engine converts heat energy into mechanical work. In this scenario, knowing the efficiency helps determine how much heat must be supplied to achieve the desired work output, considering the heat lost during the cycle.
Recommended video:
Introduction to Heat Engines