Here are the essential concepts you must grasp in order to answer the question correctly.
Thermal Expansion
Thermal expansion refers to the tendency of materials to change in size or volume in response to temperature changes. For solids, this is typically linear expansion, where the length of an object increases as its temperature rises. The amount of expansion can be calculated using the formula ΔL = αL₀ΔT, where ΔL is the change in length, α is the coefficient of linear expansion, L₀ is the original length, and ΔT is the change in temperature.
Recommended video:
Coefficient of Linear Expansion
The coefficient of linear expansion (α) is a material-specific constant that quantifies how much a material expands per degree of temperature increase. For steel, this value is approximately 11 x 10⁻⁶ /°C. Understanding this coefficient is crucial for calculating the total expansion of the steel rails when the temperature changes from winter to summer conditions.
Recommended video:
Temperature Difference
The temperature difference (ΔT) is the difference between two temperatures, which in this case is the summer temperature (33.0°C) minus the winter temperature (-9.0°C). This difference is essential for calculating the total expansion of the steel rails, as it directly influences how much space must be left between adjacent rails to accommodate the expansion without causing deformation or buckling.
Recommended video:
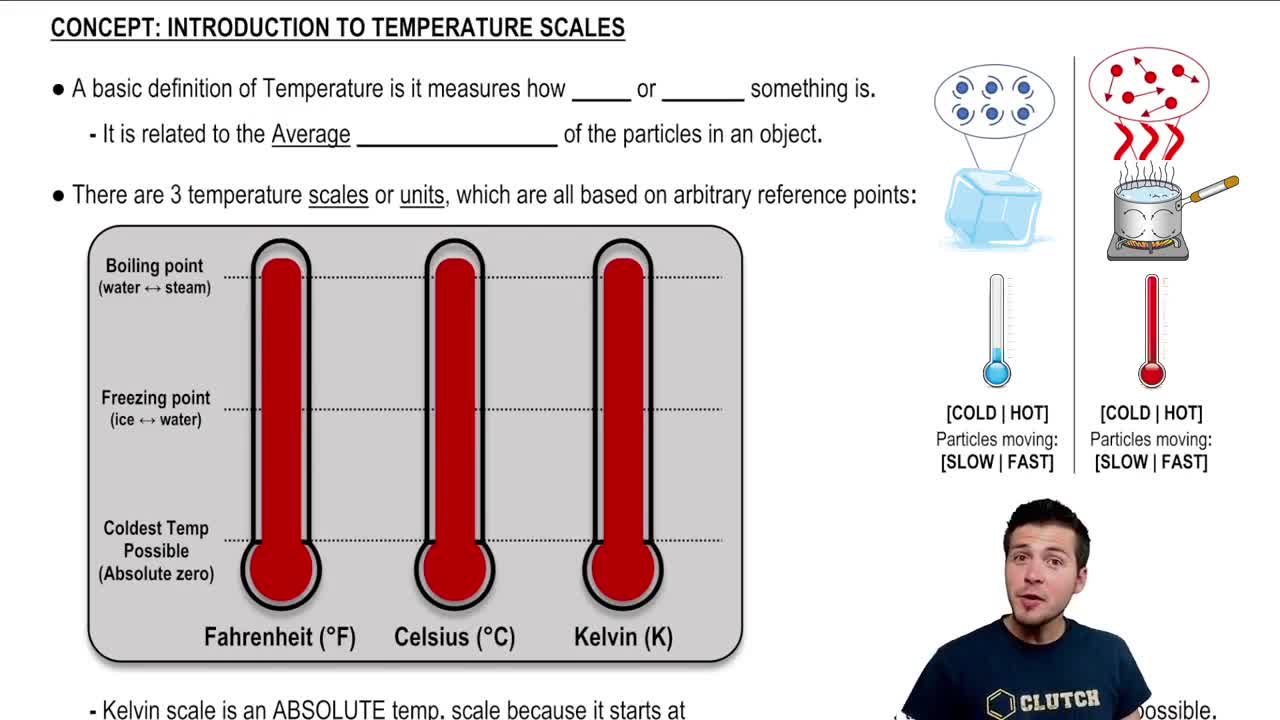
Introduction To Temperature Scales