Here are the essential concepts you must grasp in order to answer the question correctly.
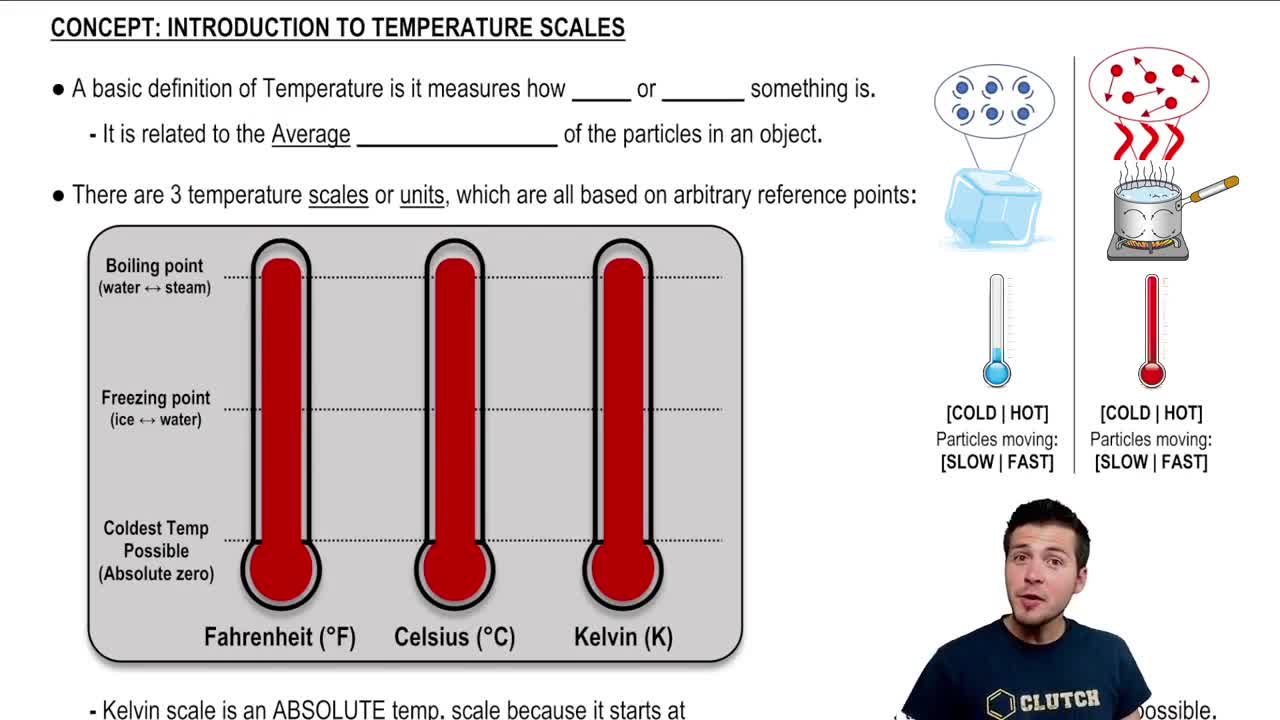
Temperature Scales
Temperature can be measured using different scales, the most common being Celsius, Fahrenheit, and Kelvin. Each scale has its own zero point and increments, which can lead to confusion when converting between them. Understanding how these scales relate to one another is crucial for solving problems involving temperature.
Recommended video:
Introduction To Temperature Scales
Conversion Formulas
To find the temperature at which Fahrenheit and Kelvin agree, one must use the conversion formulas between these scales. The formula for converting Fahrenheit (F) to Celsius (C) is F = (C × 9/5) + 32, and for Celsius to Kelvin (K), it is K = C + 273.15. These formulas allow for the necessary calculations to determine the point of agreement.
Recommended video:
Point of Agreement
The point at which two temperature scales agree is a specific temperature where their numerical values are the same. In this case, it involves finding a temperature that satisfies both the Fahrenheit and Kelvin scales simultaneously. This concept is essential for understanding how different temperature measurements can align under certain conditions.
Recommended video:
Angular Momentum of a Point Mass