In this video, we're going to take a look at the Eglinton reaction. Now, we're going to say that the Eglinton coupling reaction involves the coupling between two identical terminal alkynes with a copper catalyst and base. We want to make sure that the two alkynes we're using are identical so as to avoid a mixture of possible products. We're going to say here that the reaction uses a copper catalyst in the formation of a bialkynal product. And like coupling reactions, it has the same two driving forces. One driving force is the formation of conjugated products. Remember, the more conjugation our compound possesses, the more stable it is. And, we're going to say that another driving force is that unlike other coupling reactions, it doesn't use a catalytic cycle but instead radicals. The general setup for an Eglinton coupling reaction is that we have alkyne 1, which is identical to alkyne 2. We utilize copper 1 and copper 2 catalysts. And we have pyridine as our base. Remember, pyridine is like benzene except there's a nitrogen within it. And basically, what happens here is that we have the loss of hydrogen from both of these so that the alkyne portions that are left at the end combine together to give us our bialkynal product. We're going to look at the mechanism later on to see how it really occurs. But for simplicity's sake, you could just think of it as the two hydrogens being lost, and what is left combines together. Since our two alkynes are identical, that means that their R groups would be the same. So here we're going to say the R1 and R2 groups of the terminal alkynes can be represented by vinyl or aryl groups. They can also be represented by an alkyl group or even another alkyl group. So that's just the basic setup of the Eglinton reaction. Now that we've talked about the basic steps involved, click on to the next video and see how I approach the example question where we are asked to find the final product.
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect7h 3m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table24m
- 1H NMR:Spin-Splitting (N + 1) Rule22m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams12m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 34m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 31. Catalysis in Organic Reactions1h 30m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
- 36. Synthetic Polymers1h 49m
- Introduction to Polymers6m
- Chain-Growth Polymers10m
- Radical Polymerization15m
- Cationic Polymerization8m
- Anionic Polymerization8m
- Polymer Stereochemistry3m
- Ziegler-Natta Polymerization4m
- Copolymers6m
- Step-Growth Polymers11m
- Step-Growth Polymers: Urethane6m
- Step-Growth Polymers: Polyurethane Mechanism10m
- Step-Growth Polymers: Epoxy Resin8m
- Polymers Structure and Properties8m
Eglinton Reaction - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIThe Eglinton reaction is a coupling process involving two identical terminal alkynes, facilitated by a copper catalyst and pyridine base. The mechanism consists of four steps: deprotonation, where the terminal alkyne hydrogen is removed to form an alkynide ion; substitution, where the alkynide ion reacts with copper acetate; radicalization, leading to the formation of alkynide radicals; and dimerization, where two radicals combine to yield a stable bialkene product. This reaction emphasizes the stability gained through conjugation and the unique role of radicals instead of a catalytic cycle.
Eglinton Reaction
Video transcript
Eglinton Reaction Example 1
Video transcript
Although we haven't gone over the coupling mechanism for the Eglinton coupling reaction, we can take a simple approach to find our answer. So if we take a look at this example here, it says determine the product from the following Eglinton coupling reaction. Now, here we have 2 identical terminal alkynes. If this were an actual question, you wouldn't have the number 2 here given to you. You'd have to realize that, oh, I'm dealing with the Eglinton coupling reaction. Therefore, I'm dealing with 2 identical terminal alkynes. So it's kind of implied that there isn't just one of these alkynes, there's actually 2 of them. Now all we have to do is, we're going to draw 1 terminal alkyne, then realize that there's going to be an identical one also involved in the reaction. And in terms of simplicity, we know that through a process that we haven't seen yet, that the hydrogens that are on the terminal alkyne carbons will be lost somehow and that the alkyne carbons then would connect with one another. This would give me my final product as, okay. We'd have this terminal alkyne connecting with this terminal alkyne. So this would be our final product. Now, this reaction also involves copper 2 acetate being used here and we have pyridine base also involved in this reaction. But again, even though we haven't done the mechanism yet for this coupling reaction, we know that from what we've seen up above, the hydrogens on both ends of the terminal alkynes would have been lost and so those 2 carbons left behind would connect together to give us our bialkynyl product that we have here. Now that we've seen this simple example question, click on to the next video and see how exactly does the coupling mechanism work for the Eglinton coupling reaction.
Eglinton Reaction
Video transcript
So, the Eglinton coupling reaction can be seen as being comprised of 4 basic steps when it comes to its coupling mechanism. Step 1 involves deprotonation. Step 2 substitution. Step 3 is radicalization and finally step 4 is dimerization. Now, if we start out with deprotonation, we're going to say the slight acidity of the terminal alkyne hydrogen allows it to be deprotonated by the pyridine base. So here we have this slightly acidic hydrogen. Remember that the nitrogen within our pyridine base has a lone pair. It utilizes that lone pair to remove this hydrogen here. When it's removed, carbon holds on to the electrons within the bond. So as a result, we create an alkynide ion. Plus, as a byproduct, we make Pyridium ion as our second product. Now byproduct, we're not really going to be concerned with. Now we head into step 2, substitution. So the alkynide ion that we made in step 1, it's formed during deprotonation undergoes a substitution with copper acetate. So here, this carbon uses its lone pair to attach to carbon which causes the breaking of this bond towards the acetate ion. So, we wind up making is we have our alkyne here that is now connected to this copper. And we have as another byproduct our acetate ion. From here we go into the 3rd step which is radicalization. So the newly formed carbon-copper bond undergoes homolytic cleavage in order to form an alkynide radical. So remember, in homolytic cleavage we have equal splitting of our single bond. So this bond will split. The terminal alkyne carbon will hold on to an electron. This carbon this copper here uses this electron to actually attach itself to this acetate. Now remember, with homolytic cleavage we don't have full arrows. Instead we have half or hook arrows being used because we only have the movement of individual electrons. At the same time that this acetate uses this electron to connect to this copper, this copper in the bond takes its electron and holds on to it. So as a result, what we'll have at this point is our alkynide radical, plus 2, copper's connected to acetates. Now, finally we have dimerization. So the final step involves the dimerization of the 2 alkynide radicals that have been formed. Notice I say 2 alkynide radicals. That's because during this entire process, remember, for this coupling reaction, we're using 2 identical terminal alkynes. So all that's happening here to alkyne number 1, the same exact thing has been happening to alkyne number 2, which is why by the time we get to our 4th step, we have 2 alkynide radicals. Dimerization can be seen as a form of termination where 2 radicals join together to form a new stable compound. So here, half arrow because we're moving 1 electron, half arrow because we're moving 1 electron, and now these 2 are joined together. So this would be our final product. Coupling reactions, that you might be used to. It doesn't use a catalytic cycle in order to form the product, instead we have the utilization of radicals. As long as you can remember the basic setup for this type of coupling reaction, you can get to the product very easily. But if you need to work out the mechanism, just remember that it's comprised of 4 steps within its mechanism. We have deprotonation, substitution, radicalization, and then finally dimerization to get our bialkenal product.
Determine compounds A and B from the following reaction sequence.

Problem Transcript
Predict the product formed from the following intramolecular Eglinton reaction.
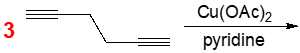
Problem Transcript
Do you want more practice?
More setsHere’s what students ask on this topic:
What is the Eglinton reaction in organic chemistry?
The Eglinton reaction is a coupling process involving two identical terminal alkynes, facilitated by a copper catalyst and pyridine base. The mechanism consists of four steps: deprotonation, where the terminal alkyne hydrogen is removed to form an alkynide ion; substitution, where the alkynide ion reacts with copper acetate; radicalization, leading to the formation of alkynide radicals; and dimerization, where two radicals combine to yield a stable bialkene product. This reaction emphasizes the stability gained through conjugation and the unique role of radicals instead of a catalytic cycle.
 Created using AI
Created using AIWhat are the steps involved in the Eglinton reaction mechanism?
The Eglinton reaction mechanism consists of four steps:
- Deprotonation: The terminal alkyne hydrogen is removed by the pyridine base, forming an alkynide ion.
- Substitution: The alkynide ion reacts with copper acetate, forming a copper-alkyne complex.
- Radicalization: The carbon-copper bond undergoes homolytic cleavage, creating alkynide radicals.
- Dimerization: Two alkynide radicals combine to form a stable bialkene product.
 Created using AI
Created using AIWhat role does the copper catalyst play in the Eglinton reaction?
In the Eglinton reaction, the copper catalyst facilitates the formation of the bialkene product. Copper (I) and copper (II) catalysts are used to assist in the substitution and radicalization steps. The copper catalyst helps in the formation of the copper-alkyne complex and the subsequent homolytic cleavage to generate alkynide radicals, which then dimerize to form the final product. Unlike other coupling reactions, the Eglinton reaction does not use a catalytic cycle but relies on radical intermediates.
 Created using AI
Created using AIWhy is pyridine used as a base in the Eglinton reaction?
Pyridine is used as a base in the Eglinton reaction because it effectively deprotonates the terminal alkyne hydrogen due to its basicity and the presence of a lone pair on the nitrogen atom. This deprotonation step is crucial for forming the alkynide ion, which then participates in the subsequent steps of the reaction. Pyridine's structure, similar to benzene but with a nitrogen atom, makes it a suitable base for this reaction.
 Created using AI
Created using AIWhat are the driving forces behind the Eglinton reaction?
The Eglinton reaction is driven by two main forces:
- Formation of Conjugated Products: Conjugated systems are more stable due to delocalization of electrons, which increases the stability of the final bialkene product.
- Radical Mechanism: Unlike other coupling reactions that use a catalytic cycle, the Eglinton reaction relies on the formation and dimerization of radicals, which drives the reaction forward.
 Created using AI
Created using AI
